Fig. 5. C3 deposition via IgG is alternative pathway-driven.
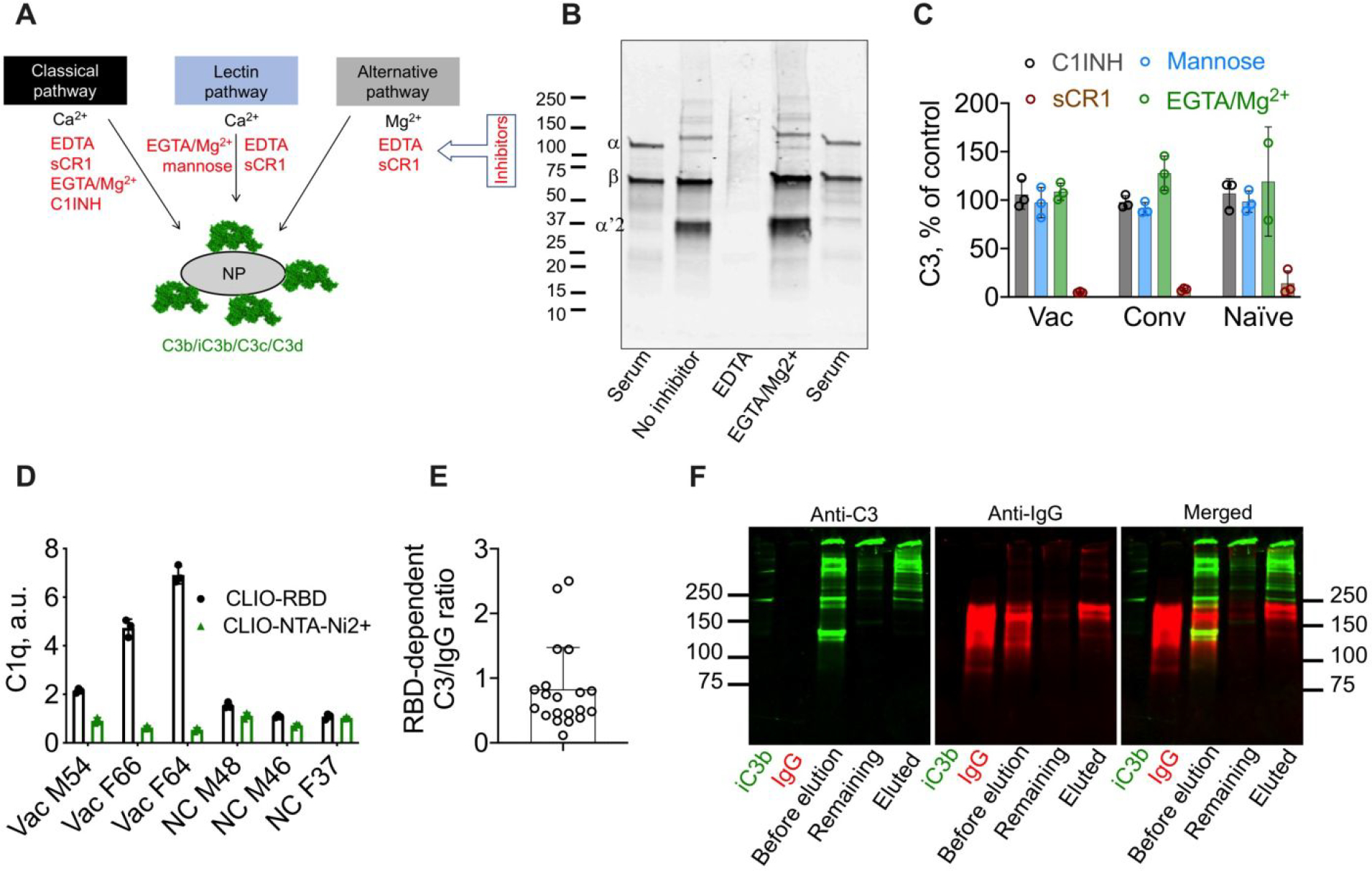
A) Three complement pathways converge into C3 cleavage and nanoparticle opsonization by C3 fragments (C3b/iC3b/C3c/C3d). Inhibitors for each pathway are shown in red; B) Western blot analysis of nanoparticle-deposited C3 in vaccinated serum. Lane 1: serum 1:200 dilution shows native C3; Lane 2: CLIO-RBD after incubation in serum; Lane 3; after incubation in serum/EDTA; Lane 4: SPIO NW after incubation in serum/EGTA/Mg2+. Intact α-chain (115kDa) and β-chain (75kDa) are detectable in serum, β-chain and α’2 (43kDa) are detectable on the particles. Some other α-chain fragments (e.g., α’1-chain) are likely to be in the high molecular weight fraction bound to other proteins via amide or ester bonds, and therefore could not be identified by their molecular weight; C) complement inhibition results (% of serum control) in donors with highest RBD-dependent C3 deposition (means of 3 donors per group, 3 technical replicates per donor) show that CP and LP are not involved in C3 opsonization. C1INH, 100μM; sCR1, 1μM; mannose, 250μM; D) dot blot analysis of binding of C1q shows increased binding to CLIO-RBD in vaccinated sera, but the binding was extremely low and did not lead to activation of the classical pathway; E) molar ratio of RBD-dependent C3 over RBD-dependent IgG deposition for vaccinated and convalescent donors shows a relatively inefficient enhancement of complement opsonization; F) analysis of association between C3 and IgG on particles in vaccinated serum (VAC M54). Proteins were eluted with 5% SDS and the eluted fraction and the nanoparticle-bound fraction were run in non-reducing SDS-PAGE and analyzed by anti-IgG/anti-C3 Western blot. C3 in the eluted fraction is mostly not associated with IgG but appears to be bound to other proteins (high molecular weight bands above 250kDa). Repeated twice.
