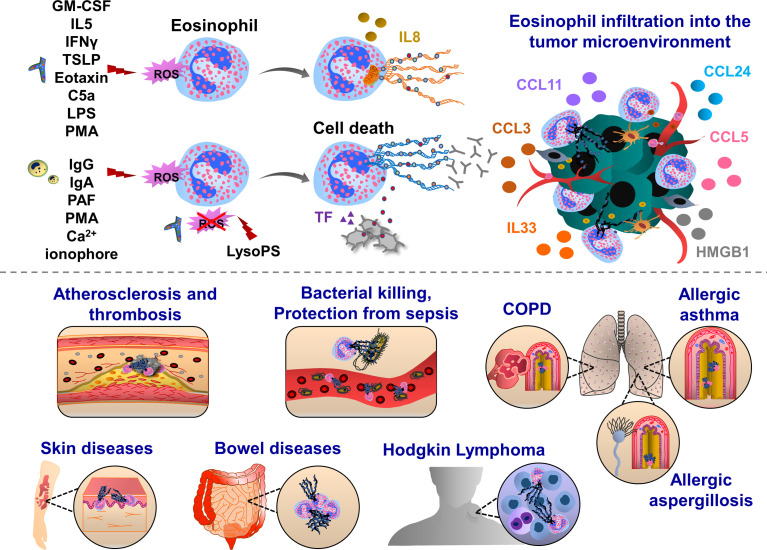
Figure 2.
Pathophysiological functions of eosinophil extracellular traps (EETs). Upon IFNγ, GM-CSF or IL5 priming, eosinophils are activated by C5a, LPS, eotaxin/CCL11, PMA, Th2 alarmin or pathogens which trigger oxidative burst and the release of mitochondrial DNA into the extracellular environment. This process can be mediated by ROS-dependent and cell death-independent pathways. In response to IgG, IgA antibodies, PAF, Ca2+ ionophore, PMA and gram-positive bacteria Staphylococcus aureus eosinophils form ETs, which ultimately induce cell death in Nox-dependent manner. Along with the chromatin, various proteins are released from activated eosinophils such as citrullinated histone 3 (orange), major basic protein (MBP, green), eosinophil cationic protein (ECP, grey) and eosinophil peroxidase (EPX, red). EETs were observed in patients with respiratory diseases, such as eosinophilic asthma, COPD and allergic aspergillosis. Eosinophil EPX triggers the production of sputum anti-EPX and anti-nuclear autoantibodies in patients with severe eosinophilic asthma, inducing resistance to the anti-asthmatic treatments. In skin diseases, EET function was often associated with host defense thereby preventing bacterial dissemination and sepsis. EETs were also observed in ruptured arterial thrombi and atherosclerotic plaques. Upon interaction with blood platelets, eosinophils form EETs and eosinophil-specific MBP released together with chromatin web-like structures activate platelets, thereby inducing the formation of thrombi. Eosinophils infiltrate various tumor types and influence tumor growth and metastasis through the interactions with endothelial cells, macrophages, fibroblasts and T cells. EETs together with NETs have been found in patients with Hodgkin’s Lymphoma displaying fibrotic and thromboinflammatory tumor microenvironment.