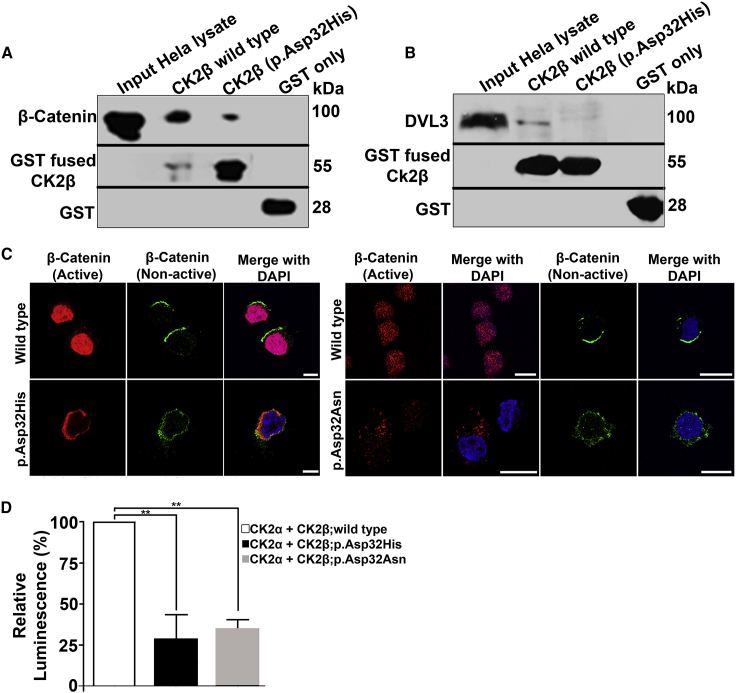
Figure 5.
Impacts of variants on the dynamics of β-catenin and DVL3
(A) Pull-down assay from HeLa total protein extracts indicates reduced interaction of endogenous β-catenin with mutant (NP_001311.3; p.Asp32His) GST-tagged CK2β as compared with wild type. GST serves as negative control. Bands of approximately 85 kDa of β-catenin were observed on western blot after probing with rabbit monoclonal β-catenin antibody. GST-fused proteins were visualized by probing the membrane with in-house-generated mouse monoclonal GST antibody.
(B) Pull-down assay shows reduced interaction of DVL3 with GST-fused CK2β mutant (NP_001311.3; p.Asp32His) as compared with wild-type. GST was used as negative control. Bands of approximately 78 kDa of DVL3 were observed by rabbit monoclonal DVL3 antibody.
(C) Immunofluorescence shows decreased amount of active β-catenin (red) in nuclei of CK2β: NP_001311.3; p.Asp32His (left panel) and CK2β: NP_001311.3; p.Asp32Asn (right panel) LCLs as compared with the wild type. Localization pattern of non-active β-catenin (green) remains the same in wild-type and both mutant LCLs. DAPI (blue) indicates staining of nucleus. Scale bar, 5 μm (left panel); 10 μm (right panel).
(D) Graph showing reduced kinase activity of CK2 carrying mutants (NP_001311.3; p.Asp32His and NP_001311.3; p.Asp32Asn) of CK2β as compared with wild type measured by ADP-Glo assay. Note that β-catenin was used as substrate. Error bars represent SD; n = 3. ∗∗p ≤ 0.01 (Student’s t test).