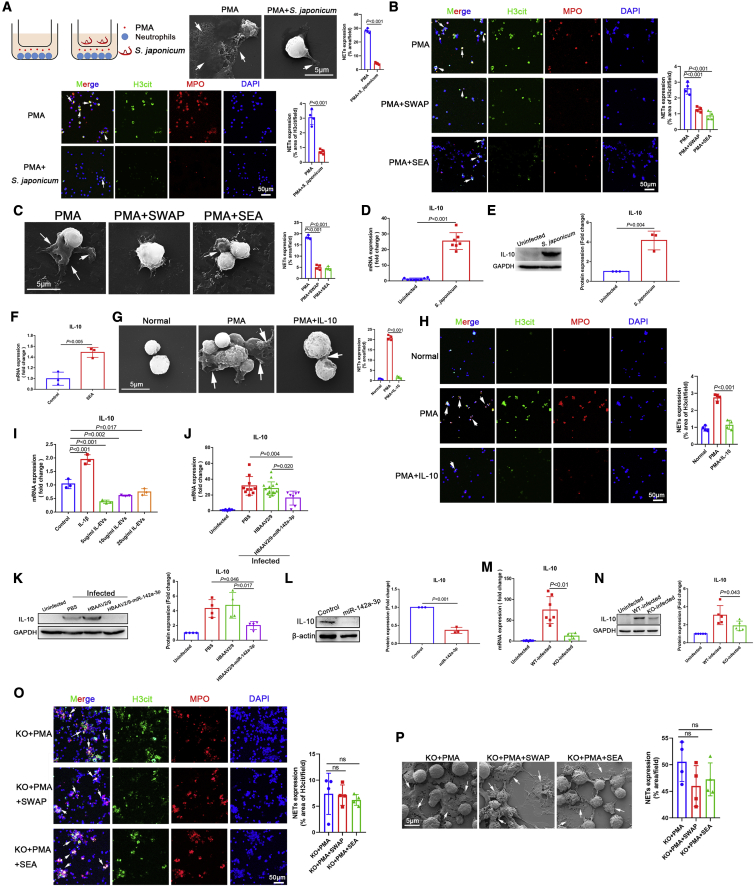
Figure 8.
S. japonicum inhibited NET formation by upregulating host IL-10 expression, whereas miR-142a-3p in IL-EVs downregulated IL-10 expression
(A) S. japonicum worms were co-cultured with PMA-simulated (500 nM, 4 h) neutrophils (5 × 105 cells) using Transwells, and NETs were observed by SEM and immunohistochemistry. (B and C) Neutrophils were treated with PMA (500 nM, 4 h), SWAP (10 μg/mL, 24 h) + PMA (500 nM, 4 h), or SEA (10 μg/mL, 24 h) + PMA (500 nM, 4 h), and NETs were observed by SEM and immunohistochemistry. (D and E) Expression of IL-10 in liver tissues was analyzed by qRT-PCR (n = 5–8 mice per group) and western blotting. (F) Macrophages were treated with SEA (10 μg/mL, 24 h), and IL-10 expression was analyzed by qRT-PCR. (G and H) Neutrophils were treated with PMA (500 nM, 4 h) or IL-10 (40 ng/mL, 24 h) + PMA (500 nM, 4 h), and NETs were observed by SEM and immunohistochemistry. (I) Macrophages were treated with IL-EVs, with IL-1β (20 ng/mL, 24 h) used as a positive control, and expression of IL-10 was analyzed by qRT-PCR. (J and K) Expression of IL-10 in the liver was analyzed by qRT-PCR (n = 7–16 mice per group) and western blotting. (L) Macrophages were treated with miR-142a-3p (50 nM, 24 h), and the expression of IL-10 was analyzed by western blotting. (M and N) Expression of IL-10 in mouse liver was analyzed by qRT-PCR (n = 5–8 mice per group) and western blotting. (O and P) Neutrophils from WASL-KO mice were treated with PMA (500 nM, 4 h), SWAP (10 μg/mL, 24 h) + PMA (500 nM, 4 h), or SEA (10 μg/mL, 24 h) + PMA (500 nM, 4 h), and NETs were observed by immunohistochemistry and SEM. (A, D, E, F, and L) Unpaired two-sample t test. (B, C, G–K, and M–P) One-way ANOVA with Dunnett’s multiple comparison test.