Fig. 2.
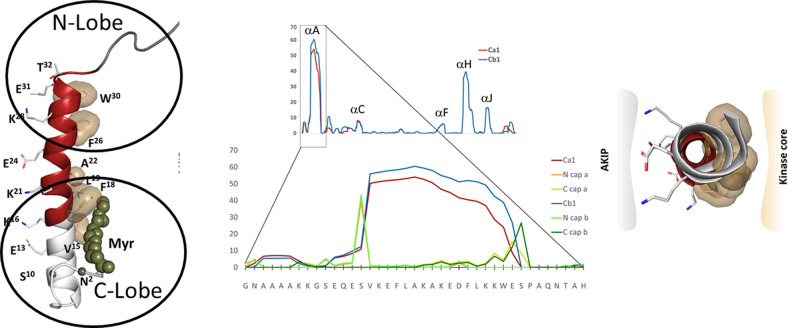
The amphipathic A-helix is a very stable protein interaction motif that is flanked by IDRs. The strong helix propensity is for the helix A is seen in the center. The A-helix (red on the left) is anchored by hydrophobic residues to the N- and C-lobes of the kinase core. The flanking region that follows the A-helix wraps around the N-lobe of the kinase core, whereas the first 14 residues encoded for by exon 1 are disordered in most crystal structures. The N-terminal myristyl group, when present, is anchored to the hydrophobic surface of the A-helix. The hydrophobic surface of the A-helix (right) binds to the kinase core, whereas the amphiphilic surface binds to AKIP1 that targets the C-subunit to the nucleus.