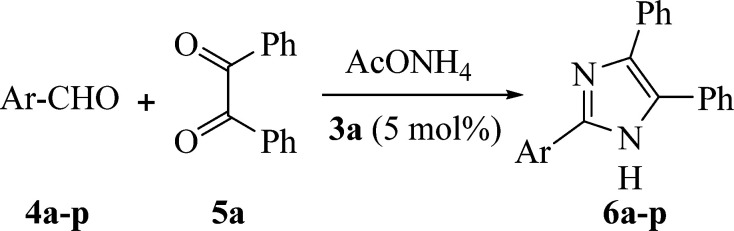
Synthesis of 2,4,5-trisubstituted imidazoles (6a–p) via the reaction of benzil (5a), aldehydes (4a–p) and NH4OAc catalyzed by 3aa.
| |||||
|---|---|---|---|---|---|
| Entry | Ar | Time (min) | Yield (%) | mp | |
| Found | Reported | ||||
| 1 | C6H5 | 5 | 99 | 272–273 | 272–274 (ref. 18) |
| 2 | 4-CH3O–C6H4 | 4 | 99 | 230–232 | 229–231 (ref. 18) |
| 3 | 4-HO–C6H4 | 4 | 98 | 234–236 | 232–234 (ref. 18) |
| 4 | 4-Me2N–C6H4 | 7 | 97 | 255–227 | 255–256 (ref. 18) |
| 5 | 2-HO–C6H4 | 6 | 95 | 202–204 | 201–203 (ref. 18) |
| 6 | 3,4-(MeO)2C6H3 | 6 | 98 | 214–216 | 213–216 (ref. 19) |
| 7 | 4-CH3–C6H4 | 8 | 95 | 230–232 | 226–227 (ref. 18) |
| 8 | 4-F–C6H4 | 8 | 96 | 260–262 | 262–264 (ref. 20) |
| 9 | 4-Br–C6H4 | 5 | 98 | 265–266 | 261–263 (ref. 21) |
| 10 | 2-Cl–C6H4 | 5 | 98 | 192–193 | 199–201 (ref. 21) |
| 11 | 4-Cl–C6H4 | 7 | 97 | 263–265 | 262–263 (ref. 22) |
| 12 | 2,4-Cl2C6H3 | 6 | 96 | 171–173 | 172–173 (ref. 20) |
| 13 | 4-NO2–C6H4 | 7 | 96 | 242–243 | 239–242 (ref. 21) |
| 14 | 2-Thienyl | 5 | 99 | 255–257 | 254–256 (ref. 18) |
| 15 | 2-Furyl | 5 | 98 | 201–202 | 201–203 (ref. 21) |
| 16 | CH3 | 6 | 99 | 244–246 | 243–245 (ref. 23) |
The mixture of 4a–p (1 mmol), 5a (1 mmol), NH4OAc (2.5 mmol) and 3a (5 mol%) in water (5 mL) was sonicated at 60 °C.
