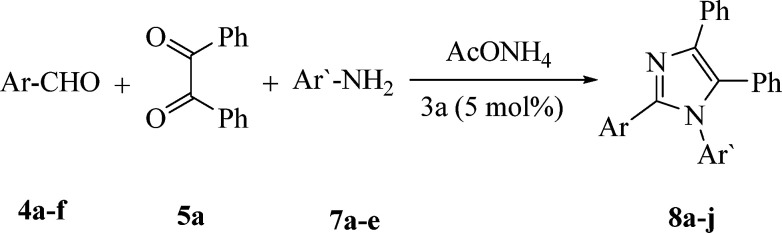
Preparation of 1,2,4,5-tetrasubstituted imidazoles (8a–j) via the reaction of benzil (5a), aldehydes (4a–f), amines (7a–e) and NH4OAc catalyzed by 3aa.
| ||||||
|---|---|---|---|---|---|---|
| Entry | Ar | Ar′ | Time (min) | Yield (%) | mp | |
| Found | Reported | |||||
| 1 | C6H5 | C6H5 | 5 | 98 | 220–222 | 219–221 (ref. 18) |
| 2 | 4-CH3–C6H4 | C6H5 | 7 | 96 | 188–189 | 185–187 (ref. 18) |
| 3 | 4-CH3O–C6H4 | C6H5 | 5 | 96 | 183–185 | 183–185 (ref. 18) |
| 4 | 4-Cl–C6H4 | C6H5 | 6 | 95 | 161–162 | 160–163 (ref. 24) |
| 5 | 4-Br–C6H4 | C6H5 | 7 | 96 | 209–211 | 207–211 (ref. 18) |
| 6 | C6H5 | C6H4–CH2 | 5 | 98 | 160–164 | 160–164 (ref. 24) |
| 7 | C6H5 | 4-CH3O–C6H4–CH2 | 5 | 98 | 150–153 | 150–154 (ref. 24) |
| 8 | 4-CH3–C6H4 | 4-CH3–C6H4–CH2 | 5 | 98 | 132–135 | 132–135 (ref. 24) |
| 9 | 4-F–C6H4 | 4-F–C6H4–CH2 | 6 | 96 | 158–161 | 158–162 (ref. 24) |
| 10 | 4-CH3O–C6H4 | 4-CH3O–C6H4–CH2 | 6 | 96 | 152–154 | 152–154 (ref. 24) |
The mixture of 4a–f (1 mmol), 7a–e (1 mmol), 5a (1 mmol), NH4OAc (1 mmol) and 3a (5 mol%) in water (5 mL) was sonicated at 60 °C.
