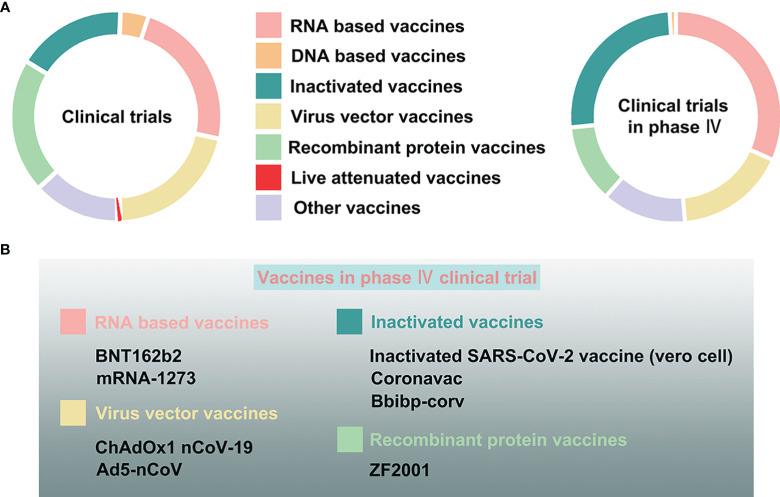
Figure 6.
The clinical trials assessing COVID-19 vaccines. (A) The proportion of varying vaccine types, namely, RNA-based, DNA-based, inactivated, live attenuated, virus vector, recombinant protein, and other vaccines, in all clinical trials, and especially in phase IV clinical trials. (B) The COVID-19 vaccines in phase IV clinical trials.