Dear Editor
Since the emergence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and its subsequent variants of concern (VOCs), the need for developing an effective vaccine having an ablility to provide immunity against different VOCs is increasing. The efforts are now directed to assess currently available vaccines or advocate the need for booster doses (third and fourth doses). Novel variants of SARS-CoV-2, such as Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529) [1,2], have the potential to exacerbate the pandemic thanks to their ability to evade immunity produced through natural infection or vaccination, and these variants might complicate the current diagnostic strategies and vaccination programs. In addition, virus mutations can affect diagnostic test accuracy. Since the appearance of the SARS-CoV-2 Omicron variant and the existence of a significant number of mutations in the variant's genome, various research groups have expressed concerns regarding the reliability of commercially and in-house designed SARS-CoV-2 specific PCR tests [3].
During the coronavirus disease 2019 (COVID-19) pandemic, many studies reported existing coinfections with SARS-CoV-2 like bacteria, fungi, and viruses [[4], [5], [6]]. SARS-CoV-2 and influenza A virus coinfections have been reported in several countries [[3], [4], [5], [6], [7]]. Seasonal influenza A virus (IAV) could exacerbate the challenges and threats that the coronavirus pandemic poses to public health, particularly during the winter months and among individuals at risk of severe disease and hospital admission. Therefore, the coinfection of these viruses could considerably influence morbidity, mortality, and the demand for healthcare services.
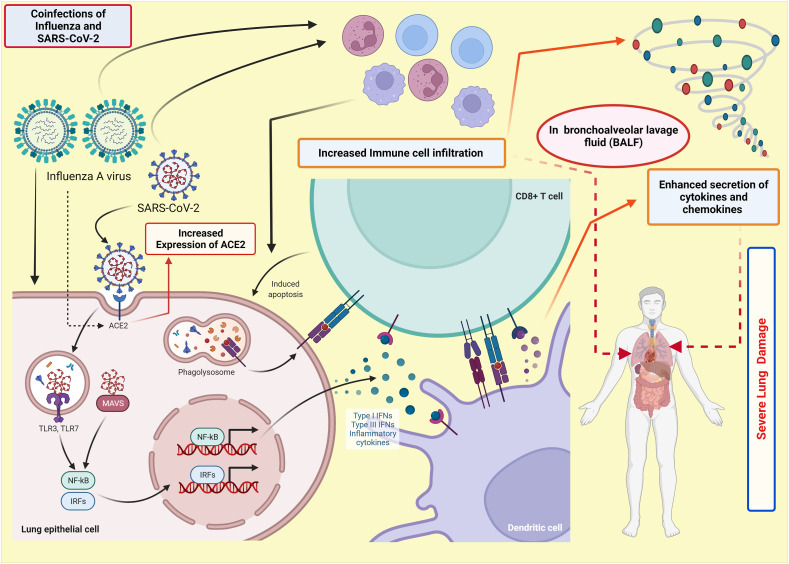
It was found in recent study that 22.3% of SARS-CoV-2 positive dead cases in Iran were coinfection with influenza virus [7]. Kim et al. [8] have used the K18-hACE2 transgenic mouse model to study the pathogenic and immunological effects of SARS-CoV-2 and IAV H1N1 coinfection. They have reported that SARS-CoV-2/IAV coinfections boosted immune cell infiltration and inflammatory cytokine levels in bronchoalveolar lavage fluid (BALF), resulting in severe pneumonia and lung injury (Fig. 1 ). The increased cytokine levels and chemokines have been postulated as a primary reason for the increased death rate in severely infected patients with COVID-19. Hence, the coinfections can significantly increase hospitalization, hence the mortality rate. Furthermore, coinfections also resulted in significant lymphopenia in the peripheral blood, with lower total IgG, neutralizing antibody titers, and CD4+ T cell responses to each virus [8]. Bai et al. [9] have reported increased SARS-CoV-2 viral load, and more severe lung damage was observed in mice coinfected with IAV. Additionally, coinfection with H1N1 and SARS-CoV-2 prolonged the duration of clinical manifestations and pulmonary damage caused by SARS-CoV-2 in ferrets [10].
Fig. 1.
Schematic representation of immunological effects of SARS-CoV-2 and IAV coinfection. IAV infection during the COVID-19 can exaggerate the immune cell infiltration in BALF (Bronchoalveolar Lavage Fluid), leading to enhanced secretion of cytokines and chemokines. In addition, IAV (Influenza A virus) infection increases the expression of ACE2 (Angiotensin-Converting Enzyme 2) receptors, which provides favourable conditions to SARS-CoV-2, which in turn enhances viral load. Hence, the coinfection of SARS-CoV-2 with the influenza virus can significantly increase the death rate.
Notably, a similar increase in SARS-CoV-2 infectivity was not seen with various other respiratory viruses, most likely due to IAV's unique ability to raise ACE2 expression [9], which could raise a question if IAV might have played a hidden role in the ferocity of COVID-19 subsequent surges (Fig. 1). Strikingly, a dramatic decrease in influenza detection has been observed since March 2020, co-incident with the exponential global spread of SARS-CoV-2. There was a 99% drop in influenza isolation over the world. Following the SARS-CoV-2 pandemic, influenza is expected to resurface and circulate once more. Addressing the COVID-19 pandemic necessitates the accurate application of diagnostic tests in large quantities, which essentially aid in implementing appropriate therapy and preventing further spread. Test availability makes up the big concern, but test accuracy may constitute a critical problem in front of the emergence of novel variants of SARS-CoV-2. To shortcut the efforts that should be done, IAV must be integrated into the COVID-19 diagnostic modalities, not for the present case but the future challenges as well.
The U.S. Food and Drug Administration (FDA) has approved two types of rapid diagnostic tests (RDTs) to diagnose SARS-CoV-2 infection: nucleic acid amplification and antigen-based immunoassay tests to detect genes and SARS-CoV-2 proteins, respectively. RDTs are valuable public health tools to detect SARS-CoV-2 variants, and they are projected to become more generally available in clinics, communities, and homes in the near future. During development and post-authorization, however, test developers and health regulators should analyse and monitor the influence of new variants on Ag-RDTs. RDTs could be used routinely for homes and places encompass gatherings like schools and universities.
Chen et al. [9] had managed to develop a dual-mode surface-enhanced Raman scattering (SERS)-based aptasensor that can diagnose and discriminate SARS-CoV-2 and influenza A/H1N1 at the same time. On an Au nanopopcorn substrate, DNA aptamers that exclusively bind to SARS-CoV-2 and IAV/H1N1 were immobilized together. SERS signals could be generated in the nanogap of the Au nanopopcorn substrate by Raman reporters (Cy3 and RRX) attached to the terminals of DNA aptamers. To eliminate errors induced by changes in the measurement environment, the internal standard Raman reporter (4-MBA) was mounted on the Au nanopopcorn substrate together with aptamer DNAs. When SARS-CoV-2 or influenza A virus approaches the Au nanopopcorn substrate, the corresponding DNA aptamer selectively detaches from the substrate due to the significant binding affinity between the corresponding DNA aptamer and the virus. As a result, the related SERS intensity decreases with increasing target virus concentration [11]. Kim et al. [12] have presented a feasible and cost-effective technique for simultaneously analyzing and identifying respiratory virus coinfections and the whole SARS-CoV-2 genome with easiness from the clinical specimens. Saviñon-Flores et al. [13] suggested that new analytical methodologies are necessary to control the adverse repercussions associated with zoonotic respiratory viruses. Multiplex assays that identify viruses along with their unique biomarkers or secondary illness biomarkers arising from infection are urgently needed at the moment.
Some researchers have emphasized the need for designing a multi-vaccine to encompass SARS-CoV-2 and IAV, or co-administering COVID-19 and Influenza vaccines, pointing out that concomitant vaccination appears to be a viable immunization strategy [14]. Co-administering the COVID-19 vaccine with seasonal influenza vaccine can be a safe and effective strategy to induce a substantial amount of immune response against both infections at the same time as it does not require time intervals in between. Recently, Toback et al. [14] did not report any early safety concerns with the concomitant administration of a COVID-19 vaccine (NVX-CoV2373) with influenza vaccines (Flucelvax Quadrivalent; quadrivalent influenza cell-based vaccine for those aged 18–64 years and Fluad; adjuvanted trivalent influenza vaccine for those 65 years or older). The dual immunization of K18-hACE2 mice with H1N1 and SARS-CoV-2 effectively prevented them from both H1N1 and SARS-CoV-2 infection [8]. After infection with H1N1 and SARS-CoV-2, all the vaccinated mice survived. Therefore, co-vaccination against H1N1 and SARS-CoV-2 could be an effective system for preventing epidemic-related pneumonia in the coming winters [10]. It was reported that concurrent seasonal influenza vaccination (quadrivalent influenza vaccine; QIV-HD: high-dose quadrivalent influenza vaccine) with a COVID-19 (mRNA-1273) vaccine booster could help to maximize protection against both infections in people at risk of serious illness and hospitalization, without noticeable serious adverse events in people aged 65 and over. Interestingly, intranasal influenza-based boost vaccination was shown to enhance mucosal and systemic immunity against SARS-CoV-2 in both the upper and lower respiratory systems, and also could boost the BioNTech vaccine for improved mucosal immunity [15]. Additionally, Bommireddy et al. [16] have designed a hybrid vaccine for SARS-CoV-2 and influenza viruses, using influenza virus-like particles (VLP) incorporated by protein transfer with glycosylphosphatidylinositol (GPI)-anchored SARS-CoV-2 S1 RBD fused to GM-CSF as an adjuvant. The hybrid vaccine elicited a high antibody response, protected mice against influenza virus and mouse-adapted SARS-CoV-2 challenges, and vaccinated mice had significantly lowered lung viral titers than naive mice.
Non-pharmaceutical interventions like social isolation, travel and movement restrictions, and hygiene measures, are largely believed to have reduced influenza circulation [17]. Early studies disclosed that SARS-CoV-2 mRNA vaccines showed that they were effective in preventing symptomatic and asymptomatic infection [18]. Additionally, three mRNA vaccine doses elicit potent variant cross-neutralization, including Omicron. However, challenges of using the mRNA vaccine technology to create a four-strain influenza vaccine should not be underestimated [17]. In both industrialized and low-resource settings, continuous global development in diagnostic test readiness is needed for quicker detection of patients, possibly at the point of care, and optimized prevention and treatment. The testing of seasonal respiratory viruses should be done in seriously sick people, as other viruses may help with patient management.
In summary, the long-term diagnostic modalities should be in our minds, and creating multivalent vaccines as well, through different strategies as a hybrid vaccine strategy to combat influenza A and SARS-CoV-2 infections is a promising approach for current and future challenges that would be caused by several pandemic viruses, such as SARS-CoV-2 and influenza viruses. Post-authorization evaluation of diagnostic tests and pharmacovigilance of medicines and vaccines are indispensable paths to ensure the accuracy of diagnostic tests, the safety and efficacy of drugs and vaccines. Designing a dual diagnostic strategy for detecting SARS-CoV-2 and influenza viruses is a potentially effective approach, based on the hypothesis that this new coronavirus may circulate in the future in a seasonal manner like other respiratory viruses, particularly endemic human coronaviruses. We advocate scientific community adopt a future-thinking strategy that yields science-based solutions for future challenges through conserving resources and shortening the battles with pandemic viruses.
Ethical approval
This article does not require any human/animal subjects to acquire such approval.
Source of funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
AbdulRahman A. Saied: Conceptualization, Data Curation, Visualization, Writing - Original Draft, Writing - review & editing. Manish Dhawan: Writing - Original Draft, Writing - review & editing Priyanka: Writing - review & editing. Om Prakash Choudhary: Supervision, Writing - Original Draft, Writing - review & editing. All authors critically reviewed and approved the final version of the manuscript.
Trail registry number
-
1.
Name of the registry: Not applicable.
-
2.
Unique Identifying number or registration ID: Not applicable.
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): Not applicable.
Guarantor
Om Prakash Choudhary, Assistant Professor (Senior Scale), Department of Veterinary Anatomy and Histology, College of Veterinary Sciences and Animal Husbandry, Central Agricultural University (I), Selesih, Aizawl-796015, Mizoram, India. Tel: +91–9928099090; Email: dr.om.choudhary@gmail.com.
AbdulRahman A. Saied, National Food Safety Authority (NFSA), Aswan Branch, Aswan, 81511, Egypt, Ministry of Tourism and Antiquities, Aswan Office, Aswan, 81511, Egypt. Email: saied_abdelrahman@yahoo.com.
Provenance and peer review
Not commissioned, internally peer-reviewed.
Data statement
The data in this correspondence article is not sensitive in nature and is accessible in the public domain. The data is therefore available and not of a confidential nature.
Declaration of competing interest
All authors report no conflicts of interest relevant to this article.
References
- 1.Choudhary O.P., Dhawan M., Priyanka Omicron variant (B.1.1.529) of SARS-CoV-2: threat assessment and plan of action. Int. J. Surg. 2022;97 doi: 10.1016/j.ijsu.2021.106187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhawan M., Priyanka, Choudhary O.P. Omicron SARS-CoV-2 variant: reasons of emergence and lessons learnt. Int. J. Surg. 2022;97 doi: 10.1016/j.ijsu.2021.106198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogels C.B.F., Breban M.I., Ott I.M., et al. Multiplex qPCR discriminates variants of concern to enhance global surveillance of SARS-CoV-2. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musuuza J.S., Watson L., Parmasad V., Putman-Buehler N., Christensen L., Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0251170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh V., Upadhyay P., Reddy J., Granger J. SARS-CoV-2 respiratory co-infections: incidence of viral and bacterial co-pathogens. Int. J. Infect. Dis. 2021;105:617–620. doi: 10.1016/j.ijid.2021.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saied A.A., Metwally A.A., Madkhal N.A., Haque S., Dhama K. Egypt's COVID-19 recent happenings and perspectives: a mini-review. Front. Publ. Health. 2021 doi: 10.3389/fpubh.2021.696082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashemi S.A., Safamanesh S., Ghasemzadeh‐moghaddam H., Ghafouri M., Azimian A. High prevalence of SARS‐CoV‐2 and influenza A virus (H1N1) coinfection in dead patients in Northeastern Iran. J. Med. Virol. 2021;93(2):1008–1012. doi: 10.1002/jmv.26364. [DOI] [PubMed] [Google Scholar]
- 8.Kim E.-H., Nguyen T.-Q., Casel M.A.B., et al. Coinfection of SARS-CoV-2 and Influenza A virus increased disease severity, impaired neutralizing antibody, and CD4+ T cell responses. J. Virol. 2022 doi: 10.1128/jvi.01873-21. jvi-01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai L., Zhao Y., Dong J., et al. Coinfection with influenza A virus enhances SARS-CoV-2 infectivity. Cell Res. 2021;31:395–403. doi: 10.1038/s41422-021-00473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao L., Deng W., Qi F., et al. Sequential infection with H1N1 and SARS-CoV-2 aggravated COVID-19 pathogenesis in a mammalian model, and co-vaccination as an effective method of prevention of COVID-19 and influenza. Signal Transduct. Targeted Ther. 2021;6:1–8. doi: 10.1038/s41392-021-00618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen H., Park S.-K., Joung Y., Kang T., Lee M.-K., Choo J. SERS-based dual-mode DNA aptasensors for rapid classification of SARS-CoV-2 and influenza A/H1N1 infection. Sensor. Actuator. B Chem. 2022;355 doi: 10.1016/j.snb.2021.131324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim K.W., Deveson I.W., Pang C.N.I., et al. Respiratory viral co-infections among SARS-CoV-2 cases confirmed by virome capture sequencing. Sci. Rep. 2021;11:1–9. doi: 10.1038/s41598-021-83642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saviñon-Flores F., Méndez E., López-Castaños M., et al. A review on SERS-based detection of human virus infections: influenza and coronavirus. Biosensors. 2021;11:66. doi: 10.3390/bios11030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toback S., Galiza E., Cosgrove C., et al. Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: an exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021 doi: 10.1016/s2213-2600(21)00409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou R., Wang P., Wong Y.-C., et al. Nasal prevention of SARS-CoV-2 infection by intranasal influenza-based boost vaccination in mouse models. EBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bommireddy R., Stone S., Bhatnagar N., et al. Influenza virus-like particle-based hybrid vaccine containing RBD induces immunity against influenza and SARS-CoV-2 viruses. bioRxiv. 2022 doi: 10.1101/2022.02.01.478657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurie K.L., Rockman S. Which influenza viruses will emerge following the SARS‐CoV‐2 pandemic? Influenza Other Respi Viruses. 2021;15:573–576. doi: 10.1111/irv.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]