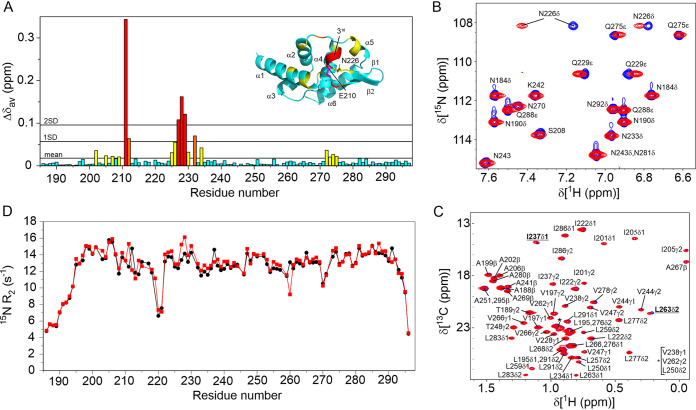
FIG 3.
NMR characterization of the effect of the phosphomimetic mutation S210E on PCTD. (A) Plot of differences to the average chemical shifts (Δδav) for the 1H15N resonances of S210E compared to wild-type PCTD. The most significant differences are for the 310 helix that includes residues of the buried C-NES. (B) An overlay of the 1H,15N HSQC of wild-type (blue) and S210E (red) PCTD that shows the significant shifts of the 1H resonances of the side chain amide of N226. (C) An overlay of the 1H,13C ct-HSQC showing the resonances of the methyls of wild-type (blue) and S210E do not significantly change supporting that the C-NES remains buried. Small shifts are observed for L263δ2 and I237δ1 which are both within 5 Å of the aromatic ring of F232 of the C-NES. (D) 15N-R2 relaxation rates of the peptide amides of wild-type (black) and S210E PCTD recorded at 15°C. No significant differences are observed, supporting that the C-NES remains buried in S210E.