Abstract
Recently it has been demonstrated that L-form cells of Proteus mirabilis (L VI), which lack a periplasmic compartment, can be efficiently used in the production and secretion of heterologous proteins. In search of novel expression systems for recombinant antibodies, we compared levels of single-chain variable-fragment (scFv) production in Escherichia coli JM109 and P. mirabilis L VI, which express four distinct scFvs of potential clinical interest that show differences in levels of expression and in their tendencies to form aggregates upon periplasmic expression. Production of all analyzed scFvs in E. coli was limited by the severe toxic effect of the heterologous product as indicated by inhibition of culture growth and the formation of insoluble aggregates in the periplasmic space, limiting the yield of active product. In contrast, the L-form cells exhibited nearly unlimited growth under the tested production conditions for all scFvs examined. Moreover, expression experiments with P. mirabilis L VI led to scFv concentrations in the range of 40 to 200 mg per liter of culture medium (corresponding to volume yields 33- to 160-fold higher than those with E. coli JM109), depending on the expressed antibody. In a translocation inhibition experiment the secretion of the scFv constructs was shown to be an active transport coupled to the signal cleavage. We suppose that this direct release of the newly synthesized product into a large volume of the growth medium favors folding into the native active structure. The limited aggregation of scFv observed in the P. mirabilis L VI supernatant (occurring in a first-order-kinetics manner) was found to be due to intrinsic features of the scFv and not related to the expression process of the host cells. The P. mirabilis L VI supernatant was found to be advantageous for scFv purification. A two-step chromatography procedure led to homogeneous scFv with high antigen binding activity as revealed from binding experiments with eukaryotic cells.
Recombinant-antibody technologies have become important for the generation of diagnostic and therapeutic molecules (13). For in vitro analysis and preclinical and clinical evaluations of selected recombinant antibodies, large amounts of highly pure and homogeneous products have to be provided, which requires high-efficiency and low-cost expression systems on a laboratory and technical scale. By overexpression of antibody constructs in the periplasmic space of Escherichia coli the heterologous protein retains its original N terminus and forms disulfide bridges. However, bacterial expression is not always the method of choice, because proteins often tend to aggregate and the expression of the antibody can lead to limited growth (13, 26, 32). A variety of other expression systems, for example, mammalian and insect cells (19), yeasts, and plants (29), have been developed. The eukaryotic systems have an efficient folding chaperone system (29) and a degradation network for unfolded by-products (18). A major drawback is the time-consuming and expensive transformation and cultivation of the eukaryotic cells.
L-form cells are stable mutants which have lost the ability to form the outer cell membrane and the murein sacculus, and they have proved to be an alternative bacterial expression system (11, 20, 21). Stable protoplasts of Proteus mirabilis, E. coli, or Streptomyces hygroscopicus are well characterized, especially with regard to their growth behavior and their membrane compositions (12, 14). Moreover, the L-form cells of P. mirabilis L VI grow not only in shaker flasks but also under semitechnical conditions, e.g., in 150-liter fermentors (10a). In this strain proteins with a signal sequence are secreted into the growth medium and their transformation and cultivation are nearly as easy, fast, and inexpensive as for E. coli strains.
Recently Kujau et al. (20) demonstrated that the L-form cells are capable of synthesizing a recombinant-antibody fragment. From that investigation they reported comparable amounts of active homodimeric miniantibody in E. coli RV308 and in the P. mirabilis L-form cell cultures (20).
Due to the fact that the expression efficiency and stability of individual single-chain variable-fragment (scFv) constructs (whose paired variable domains of heavy and light antibody chains are linked by a peptide) are strongly influenced by the amino acid sequences of their V regions, we investigated the expression of four different antibody constructs in P. mirabilis L VI in comparison to their expression in E. coli JM109 with the intention of establishing a general scFv expression system on a laboratory scale. Special attention was given to the problem of growth inhibition and protein aggregate formation, a major obstacle in prokaryotic expression of scFvs.
The four different scFv constructs are derivatives with a potential for clinical application. The first construct, scFv F19, is a derivative of a murine monoclonal antibody (MAb) which recognizes fibroblast activation protein α (FAP-α), a tumor stroma protein (9). The mammalian expression of this scFv construct gave a heterogeneous product due to glycosylation. With conventional bacterial expression systems, only a low yield of active protein (1 mg/liter) was obtained (unpublished data). The second construct, scFv OS4, is a complementary determining region grafted humanized version of scFv F19 with comparable properties. The third MAb-derived construct, scFv H398, is a human 60-kDa tumor necrosis factor receptor (TNFR60) antagonist, previously developed in our laboratory (24). It was cloned from a murine hybridoma cell line without any additional sequence modifications. The scFv H398 construct is not secretable in mammalian cells (2). When scFv H398 is expressed in E. coli JM109, expression levels of the active construct are higher and the tendency to form aggregates is less than for scFv F19 (unpublished data). The fourth construct is scFv TTX, a human, phage-display-selected antibody specific for tetanus toxoid (22a). Its scFv production in E. coli yields up to 10 mg of active protein per liter (22a). Accordingly, with these four scFv constructs we have a selection of antibodies which upon expression in E. coli differ significantly in both the yields of product and the tendency to aggregate. The data presented show that the expression of all four scFv constructs in L-form cells is superior to their expression in E. coli with regard to product yield. Further, we describe a purification procedure leading to homogeneous preparations of the bioactive protein.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and protein expression.
E. coli K-12 strain JM109 was used as the cloning and expression host. Protein expression in L-form cells was performed with P. mirabilis L VI, a stable protoplast strain, obtained from the Institut für Molekulare Biotechnologie, Jena, Germany. For growth in liquid culture, brain heart infusion (BHI) medium (Difco) supplemented with 0.5% yeast extract was used for both strains. Selection of transformants was performed with medium containing 50 mg of kanamycin per liter. For antibody expression, an overnight culture was diluted 1:100 and cells were grown in 20 to 50 ml of medium with shaking at 30°C. The protein synthesis was induced with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside) at an optical density at 550 nm (OD550) of 0.8. After the production period the supernatant was separated from the cells by centrifugation at 10,000 × g for 5 min. The cell pellet was resuspended in 1 ml of phosphate-buffered saline (PBS; pH 7.4) and sonicated. Subsequent centrifugation of the lysate at 10,000 × g for 10 min led to separation of the soluble and insoluble protein fractions of the cell proteins. For further analysis of the insoluble protein fraction, the pellet was resuspended in 1 ml of PBS. For L-form cells the active antibody was in the culture supernatant; when the antibodies were expressed in E. coli JM109, the active antibodies were mainly in the soluble protein fraction of the cell lysate. The protein content of the soluble protein fraction was determined according to a Bio-Rad protein assay, with bovine serum albumin (BSA) as the reference protein.
Cloning techniques and construction of the expression plasmid.
DNA manipulations, including plasmid isolation and molecular cloning, were performed by standard methods (22). The transformation of the L-form strain was carried out with polyethylene glycol (11). Our vectors were derived from plasmid pACK02scKan, which has been used for stable antibody expression in P. mirabilis L VI (20). The plasmid contains the expression cassette under the control of the lac promoter and an ompA signal sequence, connected to the antibody coding sequence for transport through the cytoplasmic membrane. Our first antibody, scFv H398, was integrated by overlap PCR to the ompA signal sequence with the upstream primers 5′-AAT GCA GCT GGC ACG ACA GG-3′ and 5′-GCG CAG GCC CAA GTT CAG C-3′ and the downstream primers 5′-GCT GAA CTT GGG CCT GCG C-3′ and 5′-ACC GTC ATC ACC GAA ACG CG-3′. Cloning of the resulting fragment was performed with the XbaI/HindIII restriction PCR fragment, the HindIII/BclI fragment of pOPE51 (2), and the XbaI/BamHI fragment of pACK02scKan, resulting in the vector pEA11. The introduction of an NcoI site at the cleavage signal of the signal peptidase was achieved by site-directed mutagenesis of the glutamic acid at position 20 to a methionine. The PCR primer pair was 5′-CCG CTT GCT GCA ACT CTC TC-3′ and 5′-TGC TGC AGC TGA ACT TGG GCC ATG GCT ACG G-3′. This plasmid was named pEA12 and is compatible for exchanges of the antibody cassettes to the pOPE and pSEX plasmid series (6). All other antibodies were cloned from these phage display expression vectors (6) via the NcoI and NotI restriction sites except scFv F19, which contains an internal NcoI restriction site. This antibody was cloned via PvuII/NotI restriction.
Detection of active secretion of the antibody scFv H398 in L-form cells.
In order to clarify whether translocation of the antibody constructs across the cytoplasmic membranes of the L-form cells is an active process, we used an indirect assay in which translocation is inhibited with sodium azide (3). Cells were grown and scFv H398 synthesis was induced as described above. After two generations (2.5 h), the translocation was inhibited by the addition of sodium azide at a final concentration of 0.02% (wt/vol). For analysis of the blocked translocation, cells with an OD550 of 0.05 were separated from the supernatant by centrifugation (10,000 × g, 10 min) and resuspended in loading buffer and the cell and supernatant proteins were separated by sodium dodecyl sulfate (SDS)–15% polyacrylamide gel electrophoresis (PAGE). The premature protein was detected by Western blot analysis with anti-c-myc MAb as the primary detection antibody.
Western blot analysis and determination of antibody concentration.
For Western blot analysis of scFv constructs expressed in E. coli JM109, 12 μg of the soluble protein fraction of the cell lysate and the same volume of the insoluble protein suspension were mixed with loading buffer (Roth). For analysis of the proteins expressed in P. mirabilis L VI, cell suspension at an OD550 of 0.03 (8 μg of the soluble protein fraction) and a corresponding volume of the supernatant were resuspended in loading buffer and all samples were heated at 95°C for 5 min prior to being loaded onto an SDS–15% polyacrylamide gel. Low-molecular-weight markers were supplied by Pharmacia. After electroblotting of the separated proteins onto a nitrocellulose membrane (Satorius), unspecific antibody binding was blocked with Tris-buffered saline (pH 7.5) with milk (5%, wt/vol) and Tween 20 (0.05%, vol/vol). As the primary detection antibody, an anti-c-myc-specific murine MAb (24) which recognizes a C-terminal epitope was used. For the detection of the correctly processed N terminus, we applied rabbit serum B, which binds only to the scFv when the signal sequence is cleaved of the remaining protein (5). Subsequently, an alkaline phosphatase-conjugated goat anti-mouse antibody (Dianova) or goat anti-rabbit antibody (Promega) was applied to detect the scFv H398-antibody complex with nitroblue tetrazolium and BICP (5-bromo-4-chloro-3-indolylphosphate) as the enzyme substrate. Antibody concentrations of crude extracts or culture supernatants were determined by comparison of the Western blot signal intensities to the signal intensities of a dilution series of highly purified scFv H398 on the same Western blot.
ELISA for characterization of specific antigen binding of scFv constructs on immobilized antigen.
Recombinant human TNFR60, the antigen of scFv H398, was purified from insect cell supernatant (24). Tumor antigen and tetanus toxoid were generous gifts from Boehringer Ingelheim Pharma KG, Biberach, Germany, and Behring GmbH, Marburg, Germany, respectively. The enzyme-linked immunosorbent assay (ELISA) procedure for determination of the antigen binding activity has been described recently (24). Briefly, 100-ng samples of antigen were immobilized on microtiter plates (catalog no. 655081; Greiner) in PBS at 4°C for 16 h. The microtiter plates were incubated with the following solutions: 1× Roti Block (Roth) for 1 h at room temperature, dilutions of the soluble protein fraction of lysed E. coli JM109 or supernatant of P. mirabilis L VI transformants for 2 h at room temperature, anti c-myc MAb for 16 h at 4°C, and anti-mouse antibody conjugated with horseradish peroxidase (catalog no. 515035071; Dianova) for 2 h at room temperature to detect antigen-scFv antibody complexes. Recombinant antibodies were incubated in 5% (vol/vol) fetal calf serum in PBS. All other antibodies were incubated in 0.05% (vol/vol) Tween 20 in PBS. After each incubation step, the plates were rinsed with 0.05% (vol/vol) Tween 20 in PBS and subsequently PBS alone. The colorimetric reaction was performed with ABTS [2,2′-azino-di-(3-ethyl-benzthiazolinsulfonate 6)] as the enzyme substrate.
Dilution series of supernatants and crude extracts revealed a typical saturation curve in plots of ELISA signals to volumes. The slopes in the linear regions of the plots were proportional to the concentrations of the antigen binding activity and could be compared to each other or to an aliquoted and frozen standard of known antibody concentration.
Characterization of antigen binding of scFv OS4 to eukaryotic cell lines.
Stable transfectants of HT1080 cells (human sarcoma cells, gift from Boehringer Ingelheim Pharma KG) expressing FAP-α and FAP-α− control HT1080 cells were used to investigate the cell binding of scFv OS4. Eucaryotic cells were cultivated as described previously (2). Different dilutions of scFv antibodies purified by immobilized-metal affinity chromatography IMAC or size exclusion chromatography in PFA (PBS, 2% fetal calf serum, 3 mM sodium azide) or PBS plus 2% (wt/vol) solubilized milk powder were used. The milk-blocked dilutions were centrifuged (10,000 × g, 10 min) twice, and the supernatants or the unblocked PFA dilutions were incubated with 104 HT1080 cells per well in a microtiter plate for 1 h at room temperature. Subsequently, the cell suspension was incubated with anti c-myc MAb and alkaline phosphatase-conjugated anti-mouse antibody (Dianova) for 30 min at room temperature to detect antigen-scFv antibody complexes on the cell surface. After each incubation, the cells were rinsed three times with PFA-PBS (1:1). The colorimetric reaction was performed with 4-nitrophenylphosphate as the enzyme substrate.
Determination of scFv H398 aggregation kinetics in the supernatant of P. mirabilis L VI.
ScFv H398 was produced in a 20-ml overnight culture of P. mirabilis L VI(pEA11) as described above. The supernatant of this culture was cleared by centrifugation (6,000 × g, 10 min) and sterilized by filtration (FP 030/3 filter; Schleicher & Schuell).
Cells of P. mirabilis L VI(pACK02scKan-Δab), which contain the plasmid with a total deletion of the original McPc603 antibody derivate (20) coding sequence, were grown under similar conditions. These cells were harvested by centrifugation (6,000 × g, 10 min) and resuspended in the above-described sterile supernatant to give a final OD550 of 6. In this way, completely soluble scFv H398 was combined with control cells, which did not produce any antibody. The sterile supernatant and the supernatant with the control cells were further incubated at 30°C with shaking. After different times, samples of 1 ml were harvested and centrifuged (10,000 × g, 5 min) and the supernatants were frozen in liquid nitrogen. For a comparison of the starting, maximum antigen binding activities with the activities of the incubated supernatants, samples were thawed on ice and the antigen binding activity of each sample was determined by ELISA on immobilized antigen (TNFR60) as described above. For analysis of the insoluble scFv H398, the pellet was resuspended in 1 ml of PBS and sonicated. Aliquots of 5 μl of the pellet suspension and of the culture supernatant were analyzed by Western blot analysis with anti c-myc MAb as the primary detection antibody.
Purification of scFv antibodies from P. mirabilis L VI(pEA11) growth medium.
For isolation of recombinant scFv antibodies, the corresponding L-form cells were grown as described above and the supernatant was separated by centrifugation (10,000 × g, 15 min). Prior to ultracentrifugation (40,000 rpm, Sorvall Ti-45 rotor, 1 h) the supernatant was dialyzed against PBS (pH 8) at 4°C for 16 h. After addition of imidazole (5 mM), the supernatant was applied to the IMAC column (zinc, Hi-Trap, model 17-0408-01; Pharmacia) with a flow rate of 1.5 ml/min (peristaltic pump P1; Pharmacia). Subsequently, the column was washed with buffer (50 mM sodium phosphate [pH 8], 150 mM sodium chloride, 20 mM imidazole [25 ml]) and the proteins were eluted with elution buffer (50 mM sodium phosphate [pH 8], 150 mM sodium chloride, 300 mM imidazole [10 ml]). All operations were performed at 4°C. Fractions of 1 ml were analyzed by Coomassie blue staining of an SDS–12% polyacrylamide gel and pooled according to their product contents. Scanning and analysis of the SDS-polyacrylamide gel was performed with an Elscript 400 scanner (Hirschmann).
An aliquot of 0.5 ml of the pooled IMAC-purified sample was loaded onto the size exclusion gel chromatography column (G3000PWXL, model 08021; Tosohaas), and the separation was performed with a flow rate of 0.5 ml/min (Pharmacia) at room temperature with PBS (pH 8) as the elution buffer. Fractions of 500 μl were collected and further analyzed by ELISA, SDS–12% PAGE, and subsequent Coomassie blue staining.
The protein concentrations of highly purified scFv H398 solutions were determined according to a Bio-Rad protein assay with BSA as the reference protein.
Analytical size exclusion gel chromatography.
IMAC-purified antibody fragments were separated according to their sizes with a Superdex 75 PC 3.2/30 column and the SMART system (Pharmacia) at a flow rate of 40 μl/min, with PBS as the eluent and a fraction size of 80 μl.
RESULTS
Cloning of the scFv expression plasmids.
We intended to develop an scFv expression vector for L-form cells which is compatible with a series of modular vectors routinely used for pro- and eukaryotic expression and phage display of scFvs (6). Plasmid pACK02scKan with its ompA signal sequence for the secretion of heterologous proteins has successfully been used previously for homodimeric miniantibody expression in L-form cells and in normal E. coli cells (20, 26). For one-step cloning of scFv cassettes, an NcoI site was introduced into the processing signal of the leader peptidase of pACK02scKan by PCR. This mutation in the ompA signal sequence did not influence the processing of scFv H398 [no preprotein was detectable for the expression of scFv H398 in E. coli JM109(pEA12) (data not shown)], and this sequence is homologous to the pelB cleavage signal of pectate lysase of Erwinia carotavora.
Expression of different scFvs in E. coli JM109.
When scFv H398 was produced in the periplasmic space of E. coli JM109(pEA11), the induction of the synthesis of protein in the mid-exponential phase led to a growth deficiency compared to the level of growth in uninduced culture (Fig. 1). The maximum OD550 of the induced culture was only half of the density of the control culture, and after a short productive phase the OD550 decreased. This effect is due mainly to cell lysis, as reported by Sommerville et al. (32). For the synthesis of scFv F19 or its humanized version, scFv OS4, this effect was even stronger and E. coli grew for only two more generations after induction of protein synthesis (data not shown). For both constructs premature protein could also be detected in the cell lysates (data not shown). scFv TTX expression was more tolerated by E. coli, and antibody production showed only weak influence on cell growth (data not shown).
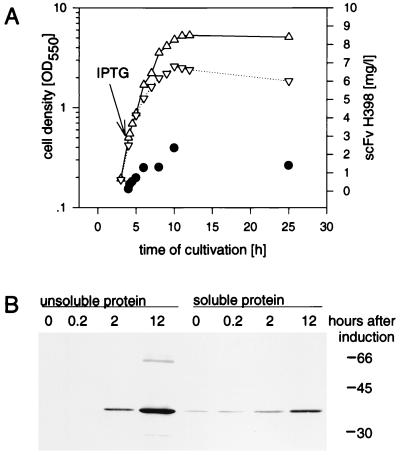
FIG. 1.
(A) Growth kinetics of E. coli JM109(pEA11) scFv H398 antigen binding activity. (B) Western blot analysis of the soluble and unsoluble proteins of E. coli JM109(pEA11) lysates. Cells were cultivated in 20 ml of medium (BHI-yeast extract-kanamycin) at 30°C, and scFv H398 synthesis was induced at an OD550 of 0.8 with 0.5 mM IPTG. At different times after induction of scFv synthesis, cells were harvested, sonicated, and fractionated by centrifugation at 10,000 × g for 10 min. The amount of active scFv H398 in the soluble protein fraction was determined by ELISA. Western blot analysis was performed as described in Materials and Methods with 12 μg of soluble cell protein or the corresponding volume of the insoluble protein suspension per lane of an SDS–15% polyacrylamide gel. The primary detection antibody was anti-c-myc MAb. ▵, uninduced culture of JM109(pEA11); ▿, induced culture of JM109(pEA11); •, active scFv H398 determined by ELISA. Molecular masses (in kilodaltons) of marker proteins are indicated at the right of the gel.
For scFv H398 very little active product was present in the soluble protein fraction (0.5% of soluble cell protein) and the major part of the scFv was found as insoluble protein in the cell lysates (Fig. 1). The amount of insoluble scFv produced in E. coli was dependent on the time of harvest, the amount of the inductor, and the strength of the promoter and comprised 95% of the total amount of scFv H398 after 12 h of induction of scFv H398 synthesis. Faint bands of higher molecular weights in the pellet fraction of the Western blot were probably dimers and multimers of scFv H398 (Fig. 1). Comparable results were observed for the other scFv antibodies. In all cases, the soluble active scFv fraction of the protein was only a minor part of the total product (data not shown).
Expression of different scFvs in P. mirabilis L VI.
The above results suggested that the periplasmic compartment of E. coli provides conditions where only a threshold concentration of scFv can be soluble. Based on the observation of Kujau et al. (20), we investigated to what extent scFv expression in cell wall-less bacteria could overcome the limitation of periplasmic expression. The induction of scFv H398 synthesis with 0.5 mM IPTG in a 20-ml shaker-flask culture of L VI(pEA11) had no influence on cell growth (Fig. 2). Similar results were obtained upon expression of the other scFv constructs, where the production of the heterologous protein also showed only minor effects on the viability of L-form cells compared to the effects on the viability of E. coli JM109.
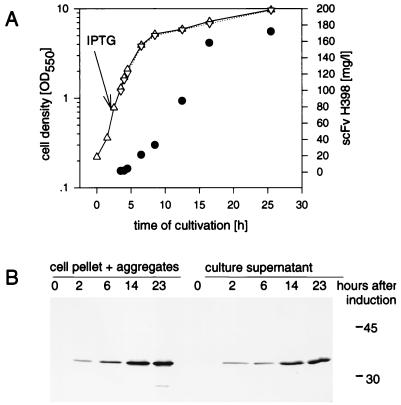
FIG. 2.
(A) Growth kinetics of P. mirabilis L VI(pEA11) and scFv H398 antigen binding activity. (B) Western blot analysis of the cell pellet and the culture supernatant. Cells were cultivated, harvested and fractionated as described in the legend to Fig. 1. The amount of active scFv H398 in the supernatant was determined by ELISA. Western blot analysis was performed as described in Materials and Methods with the cell suspension at an OD550 of 0.03 (8 μg of soluble cell protein) and with a corresponding volume of the supernatant in the lanes of an SDS–15% polyacrylamide gel. The primary detection antibody was anti-c-myc MAb. ▵, uninduced culture of L VI(pEA11); ▿, induced culture of L VI(pEA11); •, active scFv H398 determined by ELISA. Molecular masses (in kilodaltons) of marker proteins are indicated at the right of the gel.
scFv synthesis was linked to cell growth, and the product constantly accumulated in the supernatant with the highest amount of active scFv H398 forming in the early stationary phase (up to 170 mg/liter) (Fig. 2). This amount was about 9.7% of the soluble cell protein and compared to the level of activity in E. coli, represented a 15-fold increase in specific antigen binding activity (grams of scFv per gram of soluble cell protein) as determined by ELISA. A similar increase in yield was also obtained with the three other scFvs (Table 1) in L-form cells.
TABLE 1.
Yields of different scFv constructs expressed in P. mirabilis L VI and in E. coli JM109
| scFv antibody | Expression plasmid | Avg concn of scFv in L VI supernatant ± SDa (mg/liter) | Increase in yield of L VI compared to that of JM109b | Increase in specific yield of L VI compared to that of JM109c | Avg amt of purified, highly active scFv ± SDd (mg) |
|---|---|---|---|---|---|
| F19 | pEA50 | 47 ± 20 | 101 | 18 | ND |
| OS4 | pEA40 | 83 ± 23 | 160 | 32 | 11.92 ± 0.82 |
| H398 | pEA11 | 127 ± 32 | 67 | 15 | 9.20 ± 1.13 |
| TTX | pEA30 | 200 ± 20 | 33 | 17 | ND |
The contents of soluble scFvs in the supernatants of P. mirabilis L VI cultures were determined by Western blotting in four different experiments.
Values are ratios of ELISA signals per sample volumes, normalized for the volumes of P. mirabilis L VI and E. coli JM109 cultures.
Values are ratios of ELISA signals per sample volumes, normalized for the protein contents of P. mirabilis L VI and E. coli JM109 lysates.
Final yield of purified scFv from P. mirabilis L VI culture, determined by ELISA and Western blotting of two different purifications. Values are calculated for a 1-liter culture. ND, not determined.
Variations in the production conditions revealed a maximum yield of scFv H398 at a growth temperature of 30°C. At 26°C cells grew more slowly and with low productivity, and at 37°C only inactive scFv H398 could be harvested. Raising the concentration of inducer did not increase the amount of scFv H398 (data not shown). Therefore, in all experiments 0.5 mM IPTG was used. In contrast to the procedure followed by Kujau et al. (20), glucose was omitted from the medium because an acidification of the culture at early stationary phase was correlated with a decrease in the amount of active scFv H398 (data not shown).
Secretion of the scFvs is an active process of intact L-form cells.
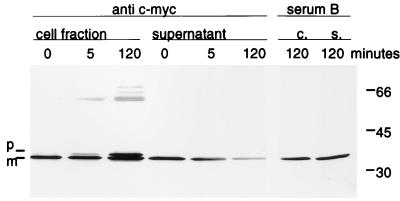
For the expression of periplasmic proteins in E. coli it has been reported that the product can also be obtained in the supernatant due to cell lysis (16). Here we demonstrate that the secretion of the scFv antibodies into the supernatant is an active Sec-dependent process linked to signal cleavage. Active transport over the cytoplasmic membrane is driven by the electrochemical gradient and coupled to ATP hydrolysis of SecA, which can be inhibited by sodium azide (4). Therefore, the addition of sodium azide to the growth medium during the early stage of scFv H398 production led to an intracellular accumulation of premature scFv within a few minutes (Fig. 3). Faint bands in the molecular-mass range of 60 kDa probably represent dimers and multimers. Even after prolonged cultivation (2 h) no further increase in extracellular scFv could be detected, thereby excluding the possibility that scFv was released due to membrane damage. Instead, we found a decline in scFv in the supernatant.
FIG. 3.
Inhibition of the scFv H398 secretion by sodium azide. scFv H398 synthesis was induced in a 20-ml shaker-flask culture of P. mirabilis LVI(pEA11) by the addition of 0.5 mM IPTG. After two generations (2.5 h), the translocation of the preprotein was inhibited by the addition of sodium azide at a final concentration of 0.02% (wt/vol). Culture aliquots (0.5 ml) were harvested at the indicated times after addition of sodium azide, and the cells were separated from the supernatant by centrifugation (10,000 × g, 10 min). Western blot analysis of the cell fraction and the supernatant was performed as described in Materials and Methods. The primary detection antibody was either anti-c-myc 9E10 or serum B, as indicated above the lanes. Molecular masses (in kilodaltons) of marker proteins are indicated at the right. c., cell fraction; s., culture supernatant; p, premature scFv H398; m, mature scFv H398.
scFv H398 secreted from L VI(pEA11) is active and soluble but has the tendency to form aggregates spontaneously under the tested culture conditions.
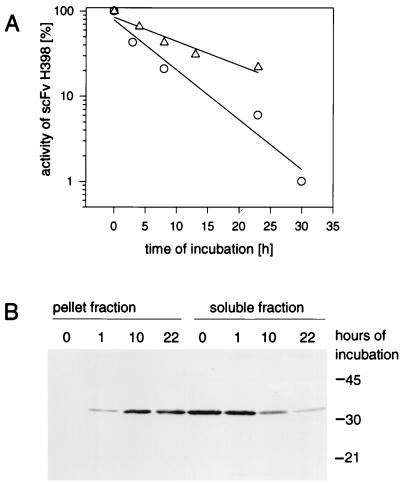
During the synthesis phase of P. mirabilis L VI(pEA11) approximately half of the scFv H398 was found to be cell associated (Fig. 2). In order to separate production from the aggregation process, we incubated the supernatant of an induced overnight culture under culture conditions with or without nonproducing cells of L VI(pACK02scKan-Δab), which carry a plasmid with a total deletion of the antibody fragment. The activity of scFv H398 decreased by first-order kinetics, with a half-life of 9 ± 1 h in the absence of cells (Fig. 4). Incubation in the presence of the cells revealed a slightly faster decay (6 ± 3 h) of the activity, which was found to depend on the integrity of the cells (unpublished observation). Fractionation of cell suspension by centrifugation and subsequent Western blot analysis revealed a decrease in scFv H398 in the soluble and an increase in the insoluble fraction during incubation (Fig. 4).
FIG. 4.
Aggregation of scFv H398 in culture supernatant. (A) Antigen binding activity. (B) Western blot analysis of aggregated scFv H398. The supernatant of an induced overnight culture of L VI(pEA11) was further incubated at 30°C under agitation with or without nonproducing P. mirabilis L VI(pACK02scKan-Δab) cells. Samples of the cell suspension or the supernatant were taken at indicated times and fractionated by centrifugation (10,000 × g, 10 min). The activity of scFv H398 in the soluble fraction was determined by ELISA. For the Western blot analysis, 5 μl of the soluble fraction and a corresponding amount of the pellet fraction from the incubation with cells were separated on an SDS–15% polyacrylamide gel. ▵, supernatant incubated without cells; ○, supernatant incubated with P. mirabilis LVI(pACK02scKan-Δab). Molecular masses (in kilodaltons) of marker proteins are indicated at the right of the gel.
Microscopic analysis by phase-contrast and indirect immunofluorescence showed the aggregates in an amorphous shape and loosely linked to or completely separated from the cells of P. mirabilis L VI(pEA11) or L VI(pACK02scKan-Δab) (unpublished results). We conclude that the aggregation of native scFv H398 in productive P. mirabilis L VI(pEA11) cultures is due to the instability of the native protein and occurs as a first-order decay reaction after the complete release and folding of the molecules.
Purification and characterization of active soluble scFv constructs.
The production and purification procedure starting from liquid culture of the corresponding L-form transformant and leading to highly homogeneous scFv was routinely performed within 2 days. The two-step purification protocol could be applied with comparable efficiencies (yields in percentages) to all four scFvs investigated. The protocol is described for scFv H398 as follows. A 50-ml shaker-flask culture (30°C) of cells producing scFv H398 was harvested at an OD550 of 6. At this point, the amount of soluble scFv was maximal whereas the amounts of other bacterial supernatant proteins were still low (Fig. 5). Dialysis and ultracentrifugation of the supernatant followed by an IMAC step resulted in an scFv H398 product of over 90% purity, with 53% recovery of the total scFv. Further analytical size exclusion gel chromatography of the affinity-purified scFv H398 sample disclosed the content of soluble high-molecular-weight scFv multimers (about 23% of the IMAC-purified soluble scFv). These multimers showed only weak binding activity in an ELISA (Fig. 6). On a preparative scale, the highly active scFv fraction of 30 kDa was isolated by the use of size exclusion chromatography, with 35% recovery of the applied IMAC-purified material. The rather low recovery of the product was due to stringent chromatography conditions and a restricted collection of those fractions containing only the monomeric scFv H398.
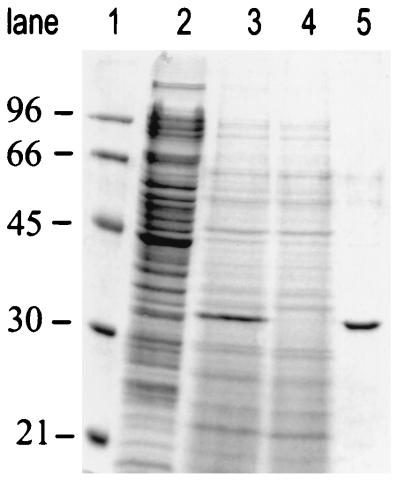
FIG. 5.
Purification of scFv H398 protein by SDS–12% PAGE in combination with Coomassie blue staining was used for analysis of scFv H398-containing samples. Lane 1 shows low-molecular-mass markers. Lanes 2 to 4 were loaded with 20-μl samples of the cell fraction, the culture supernatant with 100 mg of scFv H398 per liter and the flowthrough of the IMAC, respectively. Lane 5 was loaded with 4 μl of the pooled IMAC eluate fractions. Scanning of lane 5 revealed a purity of more than 90% for scFv H398, which is visible as a 31-kDa protein. Molecular masses (in kilodaltons) of marker proteins are indicated at the left.
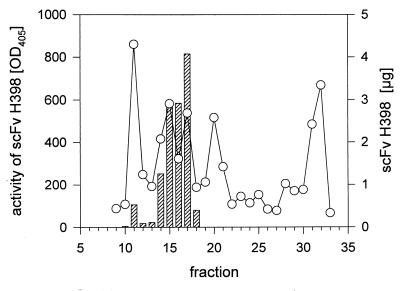
FIG. 6.
Plot of the results of analytical size exclusion chromatography of scFv H398. A 50-μl aliquot of the pooled IMAC fraction was loaded on a Superdex 75 column and analyzed by the SMART Manager version 1.31 standard protocol. Fractions of 80 μl were collected, and binding activity was analyzed by ELISA with TNFR60 as the antigen. Total scFv H398 content was determined by direct coating of the ELISA plates with the fractions. Standard proteins were BSA (67 kDa) and chymotrypsinogen (24 kDa), which eluted with fractions 9 and 13, respectively. ○, scFv H398 amount; hatched bars, antigen binding activity of scFv H398.
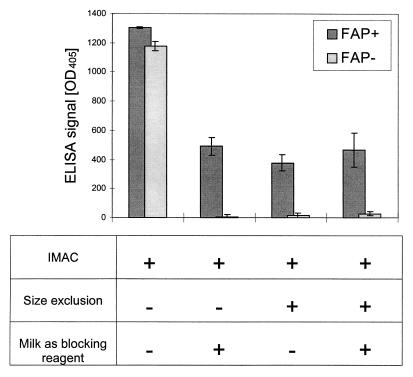
The necessity of selecting for a monomeric scFv preparation was obvious from cell binding experiments (Fig. 7) with the humanized version of scFv F19, scFv OS4. The IMAC-purified sample of scFv OS4 showed no cross-reactivity in a sensitive ELISA with immobilized antigen but revealed a strong binding reactivity not only with FAP antigen-expressing transfectants but also with the FAP-negative control transfectants (Fig. 7). This nonspecific reactivity could be diminished by excessive pretreatment with milk as the blocking reagent. However, the cross-reactivity of the scFv OS4 preparation in this cellular binding assay was completely absent after purification by size exclusion chromatography (Fig. 7), suggesting that the multimeric aggregates of scFv are responsible for the nonspecific binding to cells.
FIG. 7.
Binding activity of scFv OS4 to FAP+ and FAP− HT1080 cells. Aliquots of purified scFv OS4 after IMAC or after consecutive size exclusion chromatography were diluted to give 10- or 4-ng/μl concentrations of scFv OS4, respectively, in PFA or PBS plus milk (2%). Dilutions were chosen to give comparable signals of milk-treated IMAC and size exclusion chromatography purified samples. The scFv OS4-containing samples (75 μl) were incubated with 104 HT1080 cells with or without FAP on their cell surfaces. Specific and unspecific binding of scFv OS4 was monitored with anti-c-myc antibody as the primary detection antibody. Error bars indicate the standard errors of the means (n = 3).
DISCUSSION
In this paper we directly compared expression of scFv in E. coli JM109 and L-form cells of P. mirabilis L VI. The results showed that stable protoplast-type L-form cells overcome the typical limitations associated with periplasmic expression of heterologous proteins. These limitations are a toxic effect of the recombinant product on E. coli JM109, indicated by inhibition of culture growth to various degrees and the formation of insoluble aggregates of the protein in the periplasmic space (17, 32).
The induction of scFv synthesis with 0.5 mM IPTG led to considerable growth inhibition in all E. coli transformants studied. For example, scFv H398-producing cells reached only half of the maximum OD of the uninduced culture and further incubation led to cell lysis. Plasmid instability, i.e., loss of plasmid or sequence rearrangements, is frequently observed in heterologous gene expression (16). Consequently, for high-volume yields of scFvs heterologous protein synthesis has to be induced at high cell densities by a strictly controlled promoter system (30).
The extent of the toxicity depends on the concentration of inducer (5) and the resulting amounts of translation products as well as on the scFv sequence itself. For some scFv constructs toxicity was associated with single amino acids of the variable heavy chain (7). It is also possible that the large amount of aggregated scFv had a negative influence on E. coli growth. In contrast, for the synthesis of scFv proteins in stable protoplast-type L-form cells we found that the production of scFv H398 had a negligible influence on growth behavior (Fig. 2) for at least 10 generations (data not shown). These findings were confirmed by normal cell morphology of the productive cells by phase-contrast microscopy. Moreover, the investigation of Kujau et al. (20) revealed no plasmid instability for over 40 generations under inductive conditions without antibiotic selection, thereby clearly demonstrating the genetic stability of this L-form expression system (20).
A likely explanation for the dramatically reduced toxicity of scFv in L-form cells, compared to that in E. coli, is the dilution effect due to secretion of scFv into the surrounding medium. Therefore, cells encounter only subtoxic concentrations of the heterologous product.
In E. coli JM109 the soluble active forms of the scFv antibodies were only a minor portion of the total product. For example, more than 95% of the total scFv H398 was found as insoluble biological inactive material, depending on the time of harvest (Fig. 1). The active, soluble scFv H398 comprised only 0.5% of total soluble cell protein. It has been shown previously that a constant threshold concentration of soluble recombinant protein in the periplasm could not be influenced by promoter strength or the time of harvest but that it depended on the amino acid sequence of the expressed protein (17, 31, 34). The volume yields of soluble scFvs obtained in this study (1 to 10 mg at an OD550 of 3) are in good agreement with yields in other studies (31).
The spontaneous formation of scFv aggregates is a severe limitation in the production process. Its extent may be influenced by distinct parameters. Several groups demonstrated that slowly folding intermediates correlate with aggregation tendency because the partially folded molecules expose sufficient hydrophobic patches to allow an intermolecular interaction. If the interaction occurs with other folding intermediates, this dead-end pathway can lead to aggregated by-products (25, 33). For several scFvs it could be demonstrated that variations in their amino acid sequences revealed a higher folding efficiency and a decrease in the aggregated product (7, 16).
The observed threshold concentration of active scFv H398 in the periplasm of E. coli JM109 can also be explained as a thermodynamic equilibrium of the mono- and the multimeric forms of scFv. This explanation is based on thermal denaturation experiments (25) and on the constant occurrence of dimers and multimers of repeated scFv renaturations (16).
The production of active soluble scFv constructs in P. mirabilis L VI cultures was coupled to cell growth. Moreover, we could show that secretion is an active energy-dependent process because the inhibition of secretion by sodium azide resulted in the accumulation of unprocessed scFv H398 in the cells (Fig. 3). Final yields of active soluble scFvs with P. mirabilis L VI were in the range of 40 to 200 mg/liter in the supernatant depending on the scFv expressed (Table 1). For scFv H398 the yield of soluble protein in the culture supernatant was nearly 10% of the total soluble cell protein, which is comparable to yields of other recombinant proteins that have been produced in P. mirabilis L VI (11, 20). These results reflect at least a 15-fold higher specific activity (grams of scFv per gram of soluble cell protein) and a 67-fold higher yield in volume (grams of scFv per liter of culture) than those produced in E. coli JM109 (Table 1). The high yield of functional scFv in L-form cells is likely caused by the direct release of mature scFv into the culture supernatant. Because of the large volume of the supernatant compared to that of the periplasm (35), a single folding molecule should have enough time to complete folding properly before it interacts with another folding intermediate. In this way, L-form scFv expression may mimic the experimental renaturation of unfolded proteins via large-volume dilution (27) where the unimolecular folding reaction is completed before the multimolecular reaction of aggregation occurs (34). Moreover, the thermodynamic equilibrium between monomers and multimers should also be shifted to larger amounts of soluble monomers simply because of the higher dilution.
It is possible that scFv folding is catalyzed to a certain extent by disulfide-rearranging enzymes like DsbC (23) which might be present in trace amounts in the supernatant of L-form cells as well as by the chaperon activity which is found in membrane-bound proteins like SecD (35).
Despite the principal advantage of L-form expression, multimeric and insoluble forms of scFv H398 were also observed to a certain extent. However, this result appears to be an intrinsic feature of this particular scFv and not related to the process of expression or particular properties of the expression host. The activity of scFv H398 decayed in a first-order reaction (Fig. 4) like those of other scFv constructs (10). The increase in insoluble protein can be explained as a slow, rate-limiting, unimolecular unfolding of the functional monomer and a fast, consecutive, intermolecular aggregation reaction.
We also demonstrated that the L-form expression system is superior to the E. coli JM109 expression system not only in its higher product yields but also in its purification procedures. The use of culture supernatants reduced the amounts of those products, like cytoplasmic proteins and lipopolysaccharide, which complicate the purification of cell lysates from E. coli. Homogeneous preparations of monomeric active scFv were obtained by a consecutive combination of IMAC and size exclusion chromatography, which was shown to be essential for high antigen binding activity, especially in cell binding experiments (Fig. 7).
Although sequence modelling of scFv (25) or screening of mutated libraries (28) increased the yields of prokaryotic proteins and levels of antigen binding affinity, these methods are expensive and time-consuming and therefore are applied to special candidates only.
Here we demonstrated that P. mirabilis LVI is a broadly applicable scFv production system that even facilitates the expression of such scFvs as soluble and functionally active molecules, which are poorly produced by E. coli. A potential future use of this system is the functional expression of more-complex scFv fusion proteins (e.g., immunotoxin [8]), so far generated only by in vitro refolding of E. coli-expressed inclusion bodies.
ACKNOWLEDGMENTS
We thank M. J. Kujau for critically reading the manuscript.
This work was supported by research grants (Zentrales Schwerpunktprojekt Bioverfahrenstechnik, Universität Stuttgart, grant B 3,6 U/E) from the Bundesministerium für Bildung und Forschung and from Boehringer Ingelheim Pharma GmbH.
REFERENCES
- 1.Bowden G A, Georgiou G. Folding and aggregation of β-lactamase in the periplasmic space of E. coli. J Biol Chem. 1990;265:16760–16766. [PubMed] [Google Scholar]
- 2.Brocks B, Rode H-J, Klein M, Gerlach E, Dübel S, Little M, Pfizenmaier K, Moosmayer D. A TNF receptor antagonist scFv, which is not secreted in mammalian cells, is expressed as a soluble mono- and bivalent scFv derivative in insect cells. Immunotechnology (Amsterdam) 1997;4:173–184. doi: 10.1016/s1380-2933(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 3.Derman A I, Puziss J W, Bassford P J, Beckwith J. A signal sequence is not required for protein export in prlA mutants of E. coli. EMBO J. 1993;12:879–888. doi: 10.1002/j.1460-2075.1993.tb05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driessen A J M. How proteins cross the bacterial cytoplasmic membrane. J Membr Biol. 1994;142:145–159. doi: 10.1007/BF00234937. [DOI] [PubMed] [Google Scholar]
- 5.Dübel S, Breitling F, Klewinghaus I, Little M. Regulated secretion and purification of recombinant antibodies in E. coli. Cell Biophys. 1992;21:69–79. doi: 10.1007/BF02789479. [DOI] [PubMed] [Google Scholar]
- 6.Dübel S, Breitling F, Fuchs P, Braunagel M, Klewinghaus I, Little M. A family of vectors for surface display and production of antibodies. Gene. 1993;128:97–101. doi: 10.1016/0378-1119(93)90159-z. [DOI] [PubMed] [Google Scholar]
- 7.Forstberg G, Forsgren M, Jaki M, Norin M, Sterky C, Enhörning A, Larsson K, Ericsson M, Björk P. Identification of framework residues in a secreted recombinant antibody fragment that control production level and localisation in E. coli. J Biol Chem. 1997;272:12430–12436. doi: 10.1074/jbc.272.19.12430. [DOI] [PubMed] [Google Scholar]
- 8.Frankel A E, FritzGerald D, Siegall C, Press O W. Advances in immunotoxin biology and therapy: a summary of the Fourth International Symposium on Immunotoxins. Cancer Res. 1996;56:926–932. [PubMed] [Google Scholar]
- 9.Garin-Chesa P, Old L J, Rettig W J. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci USA. 1990;87:7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glockshuber R, Malia M, Pfizinger I, Plückthun A. A comparison of strategies to stabilise immunoglobulin Fv-fragments. Biochemistry. 1990;29:1362–1367. doi: 10.1021/bi00458a002. [DOI] [PubMed] [Google Scholar]
- 10a.Gumpert, J. Unpublished data.
- 11.Gumpert J, Cron H, Plapp R, Niersbach H, Hoischen C. Synthesis and secretion of recombinant penicillin G acylase in bacterial L-forms. J Basic Microbiol. 1996;36:89–98. doi: 10.1002/jobm.3620360205. [DOI] [PubMed] [Google Scholar]
- 12.Gumpert J, Taubeneck U. Characteristic properties and biological significance of stable protoplast type L-forms. Exper Suppl. 1983;46:227–241. doi: 10.1007/978-3-0348-6776-4_27. [DOI] [PubMed] [Google Scholar]
- 13.Hayden M S, Gilliland L K, Ledbetter J A. Antibody engineering. Curr Opin Immunol. 1997;9:201–121. doi: 10.1016/s0952-7915(97)80136-7. [DOI] [PubMed] [Google Scholar]
- 14.Hoischen C, Gura K, Luge C, Gumpert J. Lipid and fatty acid composition of cytoplasmatic membranes from Streptomyces hygroscopicus and its stable protoplast-type L form. J Bacteriol. 1997;179:3430–3436. doi: 10.1128/jb.179.11.3430-3436.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kipriyanov S M, Dübel S, Breitling F, Kontermann R E, Heymann S, Little M. Bacterial expression and refolding of single-chain Fv fragments with C-terminal cysteines. Cell Biophys. 1995;26:187–204. doi: 10.1007/BF02791580. [DOI] [PubMed] [Google Scholar]
- 16.Knappik A, Plückthun A. Engineered turns of a recombinant antibody improve its in vivo folding. Protein Eng. 1995;8:81–89. doi: 10.1093/protein/8.1.81. [DOI] [PubMed] [Google Scholar]
- 17.Knappik A, Krebber C, Plückthun A. The effect of folding catalysts on the in vivo folding process of different antibody fragments expressed in E. coli. Bio/Technology. 1993;11:77–83. doi: 10.1038/nbt0193-77. [DOI] [PubMed] [Google Scholar]
- 18.Knittler M R, Dirks S, Haas I G. Molecular chaperones involved in protein degradation in the endoplasmatic reticulum: quantitative interaction of the heat shock cognate protein BiP with partially folded immunoglobulin light chains that are degraded in the endoplasmatic reticulum. Proc Natl Acad Sci USA. 1995;92:1764–1768. doi: 10.1073/pnas.92.5.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kretzschmar T, Aoustin L, Zingel O, Marangi M, Vonach B, Towbin H, Geiser M. High-level expression in insect cells and purification of secreted monomeric single-chain Fv antibodies. J Immunol Methods. 1996;195:93–101. doi: 10.1016/0022-1759(96)00093-2. [DOI] [PubMed] [Google Scholar]
- 20.Kujau M J, Hoischen C, Riesenberg D, Gumpert J. Expression and secretion of functional miniantibodies McPC603scFvDhlx in cell wall-less L-form strains of Proteus mirabilis and E. coli. Appl Microbiol Biotechnol. 1998;49:51–58. doi: 10.1007/s002530051136. [DOI] [PubMed] [Google Scholar]
- 21.Laplace F, Müller J, Gumpert J, Malke H. Novel shuttle vectors for improved streptokinase expression in streptococci and bacterial L-forms. FEMS Microbiol Lett. 1989;65:89–94. doi: 10.1016/0378-1097(89)90371-6. [DOI] [PubMed] [Google Scholar]
- 22.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 22a.Mersmann, M., A. Schmidt, M. Tesar, A. Schöneberg, M. Welschof, S. Kipriyanov, P. Terness, M. Little, K. Pfizenmaier, and D. Moosmayer. Monitoring of scFv selected by phage display using detection of scFv-gIII fusion proteins in a microtiter scale assay. J. Immunol. Methods, in press. [DOI] [PubMed]
- 23.Missiakas D, Raina S. Protein folding in the bacterial periplasm. J Bacteriol. 1997;179:2465–2471. doi: 10.1128/jb.179.8.2465-2471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moosmayer D, Dübel S, Brocks B, Watzka H, Hampp C, Scheurich P, Little M, Pfizenmaier K. A single-chain TNF receptor antagonist is an effective inhibitor of TNF mediated cytotoxicity. Ther Immunol. 1995;2:31–40. [PubMed] [Google Scholar]
- 25.Nieba L, Honegger A, Krebber C, Plückthun A. Disrupting the hydrophobic patches at the antibody variable/constant domain interface: improving in vivo folding and physical characterisation of an engineered scFv fragment. Protein Eng. 1997;10:435–444. doi: 10.1093/protein/10.4.435. [DOI] [PubMed] [Google Scholar]
- 26.Pack P, Kujau M, Schroeckh V, Knüpfer U, Wenderoth R, Riesenberg D, Plückthun A. Improved bivalent miniantibodies, with identical avidity as whole antibodies, produced by high cell density fermentation of E. coli. Bio/Technology. 1993;11:1271–1277. doi: 10.1038/nbt1193-1271. [DOI] [PubMed] [Google Scholar]
- 27.Ruddon R W, Bedows E. Assisted protein folding. J Biol Chem. 1997;272:3125–3128. doi: 10.1074/jbc.272.6.3125. [DOI] [PubMed] [Google Scholar]
- 28.Schier R, McCall A, Adams G P, Marshall K W, Merritt H, Yim M, Crawford R S, Weiner L M, Marks C, Marks J D. Isolation of picomolar affinity anti-c-erbB-2 single-chain Fv by molecular evolution of the complementarity determination regions in the centre of the antibody binding site. J Mol Biol. 1996;263:551–567. doi: 10.1006/jmbi.1996.0598. [DOI] [PubMed] [Google Scholar]
- 29.Schouten A, Roosien J, vanEngelen F A, deJong G A M, BorstVrensssen W M, Zilverentant J F, Bosch D, Stiekema W J, Gommers F J, Schots A, Bakker J. The C-terminal KDEL sequence increases the expression level of a single-chain antibody to be targeted to both the cytosol and the secretor pathway in transgenic tobacco. Plant Mol Biol. 1996;30:781–793. doi: 10.1007/BF00019011. [DOI] [PubMed] [Google Scholar]
- 30.Skerra A. Use of the tetracycline promoter for the tightly regulated production of a murine antibody fragment in E. coli. Gene. 1994;151:131–135. doi: 10.1016/0378-1119(94)90643-2. [DOI] [PubMed] [Google Scholar]
- 31.Skerra A, Plückthun A. Secretion and in vivo folding of the Fab fragment of the antibody McPC603 in E. coli: influence of disulphides and cis-prolines. Protein Eng. 1991;4:971–979. doi: 10.1093/protein/4.8.971. [DOI] [PubMed] [Google Scholar]
- 32.Sommerville J E, Goshorn S C, Fell H P, Darveau R P. Bacterial aspects associated with the expression of a single-chain antibody fragment in E. coli. Appl Microbiol Biotechnol. 1995;42:595–603. doi: 10.1007/s002530050299. [DOI] [PubMed] [Google Scholar]
- 33.Speed M A, Wang D I C, King J. Specific aggregation of partially folded polypeptide chains: the molecular basis of inclusion body composition. Nat Biotechnol. 1996;14:1283–1287. doi: 10.1038/nbt1096-1283. [DOI] [PubMed] [Google Scholar]
- 34.Wülfing C, Plückthun A. Correctly folded T-cell receptor fragments in the periplasm of E. coli. J Mol Biol. 1994;242:655–669. doi: 10.1006/jmbi.1994.1615. [DOI] [PubMed] [Google Scholar]
- 35.Wülfing C, Plückthun A. Protein folding in the periplasm of E. coli. Mol Microbiol. 1994;12:685–692. doi: 10.1111/j.1365-2958.1994.tb01056.x. [DOI] [PubMed] [Google Scholar]