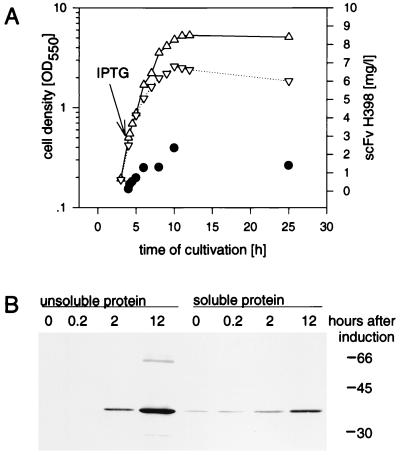
FIG. 1.
(A) Growth kinetics of E. coli JM109(pEA11) scFv H398 antigen binding activity. (B) Western blot analysis of the soluble and unsoluble proteins of E. coli JM109(pEA11) lysates. Cells were cultivated in 20 ml of medium (BHI-yeast extract-kanamycin) at 30°C, and scFv H398 synthesis was induced at an OD550 of 0.8 with 0.5 mM IPTG. At different times after induction of scFv synthesis, cells were harvested, sonicated, and fractionated by centrifugation at 10,000 × g for 10 min. The amount of active scFv H398 in the soluble protein fraction was determined by ELISA. Western blot analysis was performed as described in Materials and Methods with 12 μg of soluble cell protein or the corresponding volume of the insoluble protein suspension per lane of an SDS–15% polyacrylamide gel. The primary detection antibody was anti-c-myc MAb. ▵, uninduced culture of JM109(pEA11); ▿, induced culture of JM109(pEA11); •, active scFv H398 determined by ELISA. Molecular masses (in kilodaltons) of marker proteins are indicated at the right of the gel.