Figure 1. Endogenous and pharmacological FGF21 suppress alcohol consumption in rodents and non-human primates.
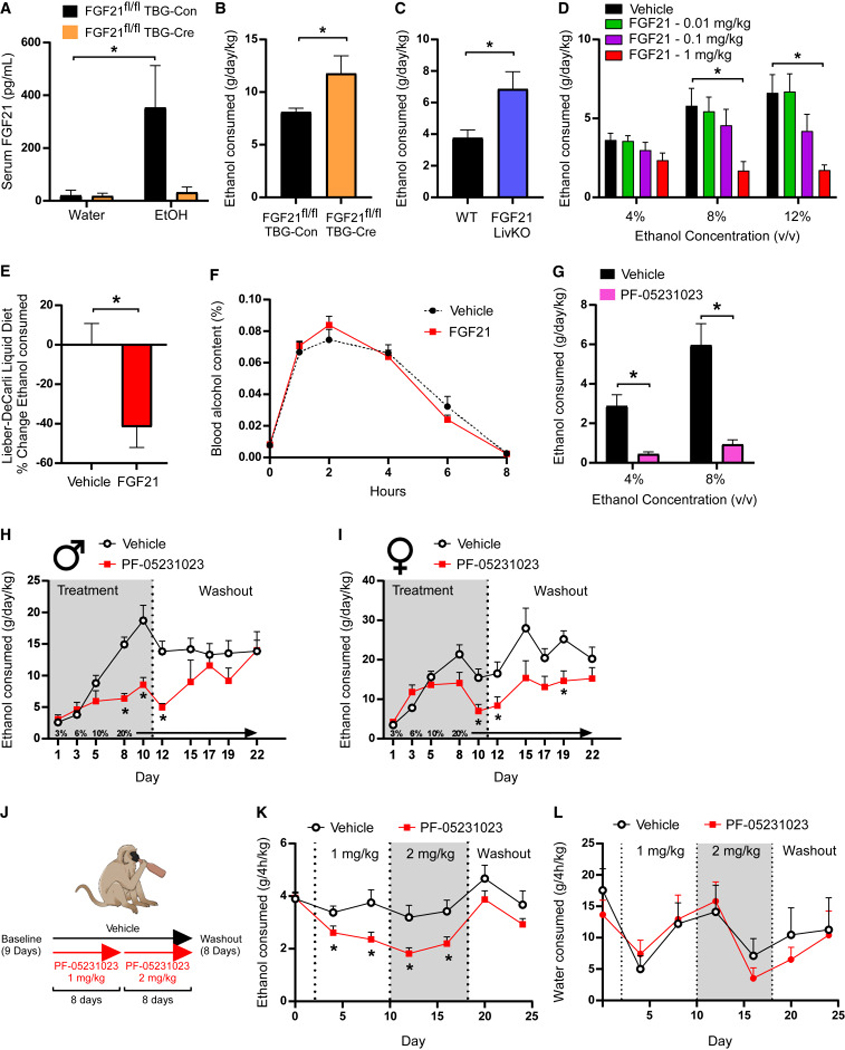
(A) Circulating levels of FGF21 in response to water or ethanol gavage (3.5g/kg) in male FGF21fl/fl mice receiving either AAV-TBG-Con or AAV-TBG-Cre (n = 6–8 mice/group, one-way ANOVA, *=P<0.05 relative to mice receiving water gavage).
(B) Ethanol (EtOH) consumption (g/day/kg) in male FGF21fl/fl mice receiving either AAV-TBG-Con or AAV-TBG-Cre allowed ad libitum access to water and EtOH using a 2-bottle choice experimental design (n = 7 and 6/group, respectively, one-tailed unpaired t-test, *=P<0.05).
(C) EtOH consumption (g/day/kg) in male FGF21 liver-specific knockout (FGF21 LivKO) mice and littermate controls allowed ad libitum access to water and EtOH using a 2-bottle choice experimental design (n = 8 and 9/group, respectively, one-tailed unpaired t-test, *=P<0.05).
(D) EtOH consumption (g/day/kg) in wildtype (WT) C57BL/6J male mice treated with the indicated doses of FGF21 (vehicle or mg/kg) via daily intraperitoneal (IP) injection across different EtOH concentrations using a 2-bottle choice experimental design (n = 6 mice/group, 2-way ANOVA w/Holm-Sidak’s multiple comparisons test, *=P<0.05 relative to vehicle treated mice for both concentrations).
(E) The percent (%) change in ethanol consumption (12%) in WT C57BL/6J male mice allowed ad libitum access to the Lieber-DeCarli liquid diet receiving daily injections of vehicle or FGF21 (IP, 1mg/kg, n = 12–13 mice/group, two-tailed unpaired t-test, *=P<0.05 relative to vehicle treated mice).
(F) Percent (%) blood alcohol content following EtOH gavage (3.5 g/kg) in WT C57BL/6J male mice treated with vehicle or FGF21 (intraperitoneal (IP), 1 mg/kg) for three days prior to EtOH gavage (n = 5 mice/group).
(G) EtOH consumption (g/day/kg) in WT C57BL/6J male mice treated with a single dose of the FGF21 analogue PF-05231023 (10 mg/kg, IP) or vehicle across different EtOH concentrations using a 2-bottle choice experimental design (n = 11 mice/group, 2-way ANOVA w/Holm-Sidak’s multiple comparisons test, *=P<0.05 relative to vehicle treated mice for both concentrations).
(H-I) EtOH consumption (g/day/kg) in WT C57BL/6J male (H) and female (I) mice treated with vehicle or PF-05231023 using the intermittent access paradigm (n = 8 mice/group, 2-way ANOVA w/Two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli, *=P<0.05 when comparing to vehicle treated mice).
(J) A schematic illustration of the experimental design used to assess effect of PF-05231023 on alcohol consumption in alcohol-preferring vervet monkeys in data presented in (K) and (L). Briefly, alcohol-preferring male vervet monkeys (n = 20) were given access to alcohol for 4 h a day for 9 days to establish baseline drinking behavior. After establishing a baseline, vervets were divided into either a placebo (n = 8) or treatment arm (n = 12). Those in the treatment arm received PF-05231023 at an initial dose of 1 mg/kg for 8 days, which was then increased to 2 mg/kg for an additional 8 days. A washout period in which no drug was administered was then performed over 8 days.
(K-L) EtOH consumption (K) and water intake (L) in the indicated treatment groups (2-way ANOVA w/Two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli, *=P<0.05 when comparing to placebo treated animals).
Data are presented as mean ± S.E.M.
