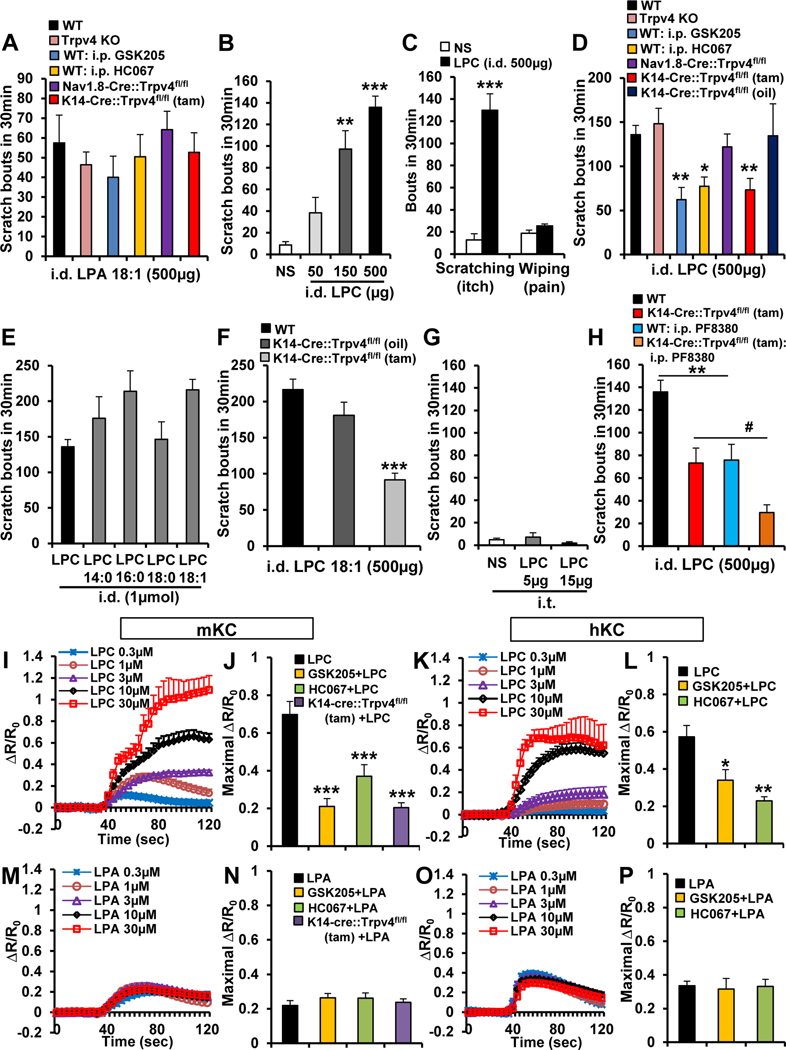
Figure 1.
Scratching behavior induced by LPC, but not LPA, requires Trpv4 in skin keratinocytes. (A) LPA(18:1)-induced itch was not significantly altered in mice: Trpv4 KO, WT intraperitoneally (i.p.) pretreated with TRPV4 inhibitors GSK205 (20 mg/kg) or HC067047 (20 mg/kg), sensory neuron-Trpv4 cKO (Nav1.8-Cre::Trpv4fl/fl), or keratinocyte-Trpv4 cKO (K14-Cre::Trpv4fl/fl, tam-inducible (n = 4–7 mice/group). i.d., intradermal. (B) LPC (egg-LPC) elicited dose-dependent scratching. **P < .01 and ***P < .001 vs normal saline (NS) (n = 5 mice for NS and 50 μg, 6 for 150 μg, and 11 for 500 μg). (C) Note LPC induced robust scratching (itch), not wiping (pain) response in the mouse cheek model. ***P < .001 vs NS (n = 4–5 mice/group). (D) LPC induced itch that was not significantly attenuated in Trpv4 KO mice or in sensory neuron-Trpv4 cKO mice but was significantly reduced in WT mice i.p. pretreated with GSK205 (20 mg/kg) or HC067 (20 mg/kg) and in keratinocyte-Trpv4 cKOs. *P < .05 and **P < .01 vs WT LPC (n = 7–11 mice/group). (E) Different species of LPC also evoked robust scratching, with LPC(18:1) most potent (n = 5 mice/species except n = 11 for LPC). (F) LPC(18:1)-evoked scratching was also significantly reduced in keratinocyte-Trpv4 cKO mice. ***P < .001 vs WT (n = 4–5 mice/group). (G) Intrathecal (i.t.) injection of LPC did not elicit scratching (n = 5 mice/group). (H) LPC-induced itch was attenuated in WT mice by i.p. pretreatment with autotaxin inhibitor PF8380 (10 mg/kg), and this attenuation was further augmented in keratinocyte-Trpv4 cKO mice. **P < .01 and #P < .05 (n = 8–11 mice/group). (I–L) LPC-induced Ca2+ influx in a dose-dependent manner in (I) mouse keratinocytes (mKCs) and (K) human keratinocytes (hKCs). R/R0 indicates the fraction of the increase of the ratio over the baseline ratio divided by baseline ratio. (J and L) LPC-induced Ca2+ influx was significantly reduced by GSK205 or HC067047 (10 μmol/L) and in (J) KC from KCTrpv4 cKO mice. *P < .05, **P < .01 and ***P < .001 vs LPC (n ≥ 180 cells recorded/treatment). (M–P) LPA(18:1)-induced Ca2+ influx was not dose-dependent and was less robust than that of LPC in (M) mouse and (O) human KCs. In addition, LPA (10 μmol/L)-induced Ca2+ signal remained unchanged with (N and P) GSK205 or HC067047 pretreatment (10 μmol/L) or in (N) keratinocytes from keratinocyte-Trpv4 cKO mice (n ≥ 200 cells recorded/treatment). Two-tailed t test for C and 1-way analysis of variance with Tukey’s post hoc test for the rest. The error bars show the standard error of the mean.