Abstract
The choroid plexus (ChP) is a major source of cerebrospinal fluid (CSF) production, with a direct and indirect role in protein clearance, and pathogenesis of Alzheimer’s disease (AD). Here, we tested the link between the ChP volume and levels of CSF proteins in 2 data sets of (i) healthy controls, mild cognitive impairment (MCI), and AD patients from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (N = 509), and (ii) healthy controls and Parkinson’s disease (PD) patients from the Parkinson’s Progression Markers Initiative (N = 302). All patients had baseline CSF proteins (amyloid-β, total and phosphorylated-tau and α-synuclein (only in Parkinson’s Progression Markers Initiative)). ChP was automatically segmented on 3T structural T1-weighted MRIs. We found negative associations between ChP volume and CSF proteins, which were stronger in healthy controls, early-MCI patients, and PD patients compared with late-MCI and AD patients. Further grouping of patients of ADNI dataset into amyloid-positive and amyloid-negative based on their florbetapir (AV45) PET imaging showed that the association between ChP volume and CSF proteins (t/p-tau) was lower in amyloid-positive group. Our findings support the possible role of ChP in the clearance of CSF proteins, provide evidence for ChP dysfunction in AD, and suggest the need to account for the ChP volume in future studies of CSF-based biomarkers.
Keywords: Choroid plexus, CSF proteins, CSF clearance, Alzheimer’s disease, Parkinson’s disease
1. Introduction
Many neurodegenerative disorders are pathologically characterized by the aberrant deposition of protein aggregates within the brain parenchyma. In Alzheimer’s disease (AD), amyloid-β (Aβ) plaques and neurofibrillary tangles are the pathognomonic pathological features (Perl, 2010). Historically, protein deposition was attributed to the increased production of aberrant proteins in the brain; however, recent studies have found that dysfunctional brain clearance systems contribute significantly to protein deposition. Compared to healthy controls, AD patients have a lower amount of Aβ clearance, while the amount of Aβ production is not different (Mawuenyega et al., 2010). This has prompted investigations on the various brain clearance systems, with possible therapeutic implications in AD (Tarasoff-Conway et al., 2015). Many of the newly found clearance systems, such as the glymphatic system, require cerebrospinal fluid (CSF) for their functionality (Jessen et al., 2015). Thus, derangements in CSF production can potentially contribute to protein deposition in neurodegenerative disorders.
Most CSF is produced in a delicate epithelial-endothelial structure, called choroid plexus (ChP) (Damkier et al., 2013), which extends along the floor of the lateral ventricles and the roof of the third and fourth ventricles (Fig. 1A and B) (Benarroch, 2016). In addition, ChP can directly clear CSF proteins via rich transporters and receptors lining its epithelial surface (Alvira-Botero and Carro, 2010; Crossgrove et al., 2005; Fujiyoshi et al., 2011). This suggests both an indirect and a direct role of ChP in the pathogenesis of neurodegenerative disorders such as AD. Animal studies have shown ChP pathological changes in AD, possibly interfering with its function in CSF production and brain clearance (Alvira-Botero and Carro, 2010). Investigating the ChP structure and function can provide insight into its possible role in protein clearance and pathogenesis of neurodegenerative disorders.
Fig. 1.
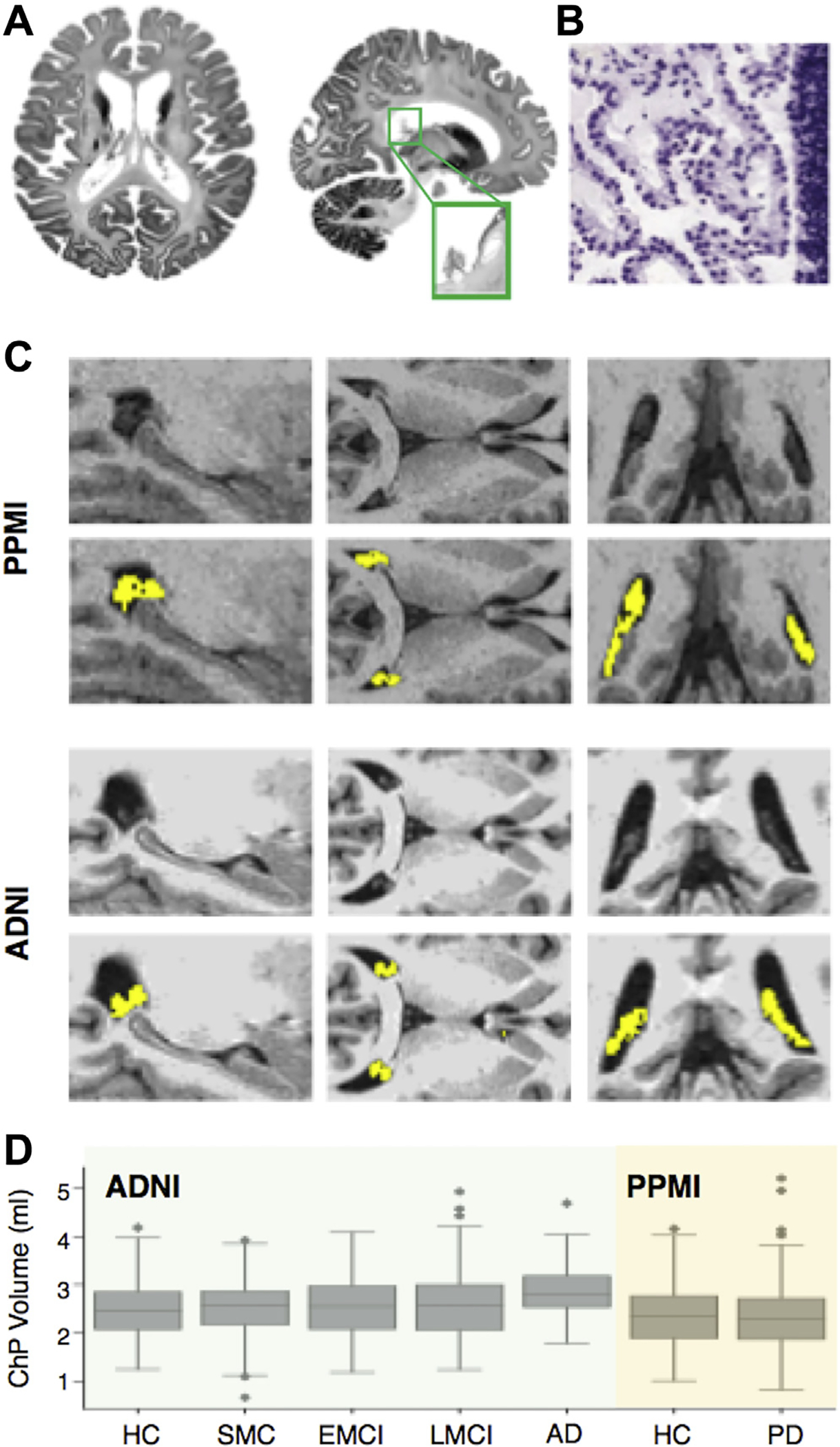
ChP segmentation. (A) Brain axial and coronal slices show ChP within lateral ventricles (left and middle panels). (B) ChP is characterized by a monolayer of epithelial cells around a stroma with blood vessels (data from Allen Brain Human Atlas; http://www.brain-map.org/). (C) ChP segmentation (yellow color) in two representative subjects of ADNI and PPMI data sets (D) ChP volume for each diagnostic group in the ADNI (green) and PPMI (orange) cohorts. We performed one-way ANOVA followed by Tukey test to examine difference in ChP volume across diagnostic groups across both cohorts. AD showed larger ChP volume compared with other groups (p < 0.05). Abbreviations: AD, Alzheimer’s disease; ADNI, Alzheimer’s Disease Neuroimaging Initiative; ChP, choroid plexus; EMCI, early-mild cognitive impairment; HC, healthy controls; LMCI, late-mild cognitive impairment; PD, Parkinson’s disease; PPMI, Parkinson’s Progression Markers Initiative; SMC, significant memory concern. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Assuming larger ChP is associated with higher CSF production as well as larger number of CSF-blood protein transporters, we first sought to assess if a negative association between the ChP volume and levels of CSF proteins can be found. We sought to examine this hypothesis in 2 large cohorts of (i) healthy controls, mild cognitive impairment (MCI), and AD and (ii) healthy controls and PD. CSF proteins have been increasingly used as diagnostic and prognostic biomarkers in various neurological disorders as they reflect the underlying brain pathology. In AD, the core CSF proteins’ profile includes Aβ, total-tau (t-tau), and phosphorylated-tau (p-tau), which reflect key aspects of disease pathogenesis, including extracellular Aβ plaque deposition, intracellular tangle formation, and neuronal degeneration (Blennow et al., 2010). While changes in t-tau and p-tau levels occur late in AD, changes in Aβ occur early in the course of the disease; therefore, Aβ changes can potentially reflect clearance dysfunction in MCI/AD (Jack and Holtzman, 2013). CSF proteins are also actively being investigated in Parkinson’s disease (PD) (Andersen et al., 2017). In addition to the core AD CSF proteins, CSF α-synuclein (α-syn) has been studied as a potential diagnostic and prognostic biomarker of PD (Hong et al., 2010). We first assessed the association between the ChP volume and each CSF protein separately. As the levels of different CSF proteins are correlated, we also used a statistical approach to find an association between the ChP volume and the correlated variation among CSF proteins. Next, we examined whether the association between the ChP volume and CSF proteins would be lower in AD compared with other diagnostic groups. Moreover, as the accumulation of Aβ occur early in the course of AD, heralding a possible clearance dysfunction, we grouped participants into amyloid-positive and amyloid-negative based on their florbetapir positron emission tomography (PET) imaging to test whether the relation between the ChP volume and CSF proteins would be lower in amyloid-positive group. Details on the study design, data, and methods are provided, with a discussion on the potential implications of the present findings and future directions.
2. Methods
2.1. Study design
We examined the link between the ChP volume and CSF protein levels in 2 large cohorts. The first cohort included healthy controls, patients with significant memory concern (SMC), early mild cognitive impairment (EMCI) patients, late mild cognitive impairment (LMCI) patients, and AD patients from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. The ADNI was launched in 2003 as a public-private partnership, led by the principal investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. Our study population consisted of healthy controls, individuals with SMC (which were combined with healthy controls throughout the study), EMCI patients, LMCI patients, and AD patients from ADNI-2 who had baseline high-resolution 3T structural T1 MRIs and CSF measures (t-tau, p-tau, and Aβ). Data used in this study were obtained from adni.loni.usc.edu.
The second cohort included healthy controls and patients with PD from the Parkinson’s Progression Markers Initiative (PPMI) data set. PPMI is a collaborative clinical and biomarker study of patients with PD and healthy controls. Our study population consisted of healthy controls and newly diagnosed, treatment-naïve PD patients who had baseline 3T structural T1-weighted MRIs and CSF measures (t-tau, p-tau, Aβ, and α-syn). For further information on the study, visit www.ppmi-info.org.
2.2. Imaging data and analysis
2.2.1. Structural T1-weighted MRIs
3T T1-weighted MRIs were downloaded from ida.loni.usc.edu website for PPMI and ADNI-2 cohorts. For a detailed description of MRI protocols, see ppmi-info.org and adni.loni.usc.edu. Automated segmentation of ChP within the lateral ventricles was performed using FreeSurfer software package (http://surfer.nmr.mgh.harvard.edu/), which has been previously utilized for segmenting ChP from structural MRIs (Baker et al., 2017; Zhou et al., 2015). We used the FreeSurfer subcortical segmentation output aseg.mgz for further analyses. We combined voxels segmented as left and right ChP (indexed as 31 and 63 in aseg.mgz) to get a single ChP mask. To ensure that the association between ChP volume and CSF proteins is not driven by other confounding factors, we also measured ventricular volume (by combining left and right lateral ventricles [aseg indices: 4 and 43], left and right inferior lateral ventricles [aseg indices: 5 and 44], third ventricle [aseg index: 14] and fourth ventricle [aseg index: 15]), as well as cortical gray matter volume (aseg indices: 3 and 42).
2.2.2. PET imaging
For florbetapir (AV45) PET data, we used average AV45 standard uptake value ratio (SUVR) of frontal, anterior cingulate, precuneus, and parietal cortex relative to the cerebellum provided by the ADNI (https://ida.loni.usc.edu/). We grouped participants into amyloid-positive and -negative using SUVR ≥ 1.11 (Landau et al., 2013; Schreiber et al., 2015) (See also Supplementary Fig. 3).
2.3. CSF proteins
In the ADNI-2 cohort, CSF was collected from all patients at the baseline and every two years. Only CSF proteins measured at the baseline were used for the purpose of this study. CSF Aβ, t-tau, and p-tau (phosphorylated at the threonine 181 position) were measured using electrochemiluminescence immunoassays on a fully automated Elecsys cobas e 601 instrument at the UPenn/ADNI Biomarker Laboratory. For this study, we used data from UPENN-BIOMK9_04_19_17.csv available on LONI website.
In the PPMI cohort, CSF was collected from all patients at the baseline and every six months. Only CSF proteins measured at the baseline were used for the purpose of this study. Three CSF biomarkers including Aβ, t-tau, and p-tau (phosphorylated at the threonine 181 position) were measured using the multiplex xMAP Luminex platform and Innogenetics immunoassay kits. CSF α-syn was measured using a commercially available enzyme-linked immunosorbent assay kit (Covance). Detailed description regarding CSF preparation and analysis can be found at ppmi-info.org.
Levels of CSF proteins are correlated with each other. To test whether ChP volume contributes to the shared variance between CSF proteins, we applied principal component analysis (PCA) to capture the correlated variation in CSF proteins. PCA is a statistical technique that decomposes the data into uncorrelated components. Each component is a weighted sum (loadings) of initial features (here, CSF proteins). The first principal component (PC1) captures the highest amount of variance, whereas the second component is orthogonal (uncorrelated) to the first component and accounts for the second-largest amount of variance, and so on. We tested the association between ChP volume and PC1 scores as it accounted for a large portion of variance in CSF proteins.
2.4. Statistical analysis
Histograms of CSF protein levels were visually inspected. Owing to the highly skewed distribution of CSF proteins, all CSF protein levels were log-transformed for both the ADNI and PPMI cohorts. We tested the association between CSF proteins (dependent variables) and age, sex, APOEε4 status, diagnostic group, cortical volume, ventricular volume, and the ChP volume in univariate linear regression analyses and multivariate linear regression analyses. We also performed 2 additional multiple regression analyses to control for total brain volume (TBV). The first analysis included TBV as a covariate and the second analysis normalized brain volumes by TBV. The results for these 2 models can be found in Supplementary Tables 2 and 3. To check for the assumptions of linear regression and potentially rectify them, we visually inspected the 4 main plots of (i) residuals versus fitted (to check for linear assumption), (ii) normal Q-Q plot (to check for normality), (iii) scale-location plot (to check for homoscedasticity), and (iv) residuals versus leverage plot (to check for influential outliers using Cook’s distance). We also used the Shapiro test to check for normality of residuals. We did not find any violation of linear regression assumptions and none of the models were influenced by an influential outlier (measured by Cook’s distance). For multiple regression models, we used the variance inflation factor (VIF) to check for possible collinearity (VIF >4 was considered as possible collinearity). PCA was applied to the CSF proteins in each data set and the scores were computed by projecting data onto the corresponding component. Partial correlation between ChP volume and PC1 was used while we controlled for the confounding covariates. The amount of variance in CSF proteins explained by ChP volume was measured in each diagnostic group separately by comparing the R2 of the model that included age, sex, APOEε4, and cortical volume (ChP- model) with the model that included age, sex, APOEε4, cortical volume, and ChP volume (ChPþ model). F-test was used to compare the 2 models (p < 0.01 was deemed statistically significant). 1000 bootstrap samples were generated (with replacement) and the change in R2 (ΔR2) was measured between ChP+ and Ch− models across all the samples to find the confidence interval for ΔR2. To test whether being amyloid-positive would change the association between ChP volume and t-tau/p-tau, we fitted a linear regression model including age, sex, APOEε4, cortical volume, ventricular volume, ChP volume, amyloid-positive, and the interaction terms between amyloid-positive and brain volumes (cortical volume, ChP volume, ventricular volume). We tested whether the amyloid-positive and ChP volume interaction was significant. For the purpose of visual presentation, we used partial correlation to show the correlation between ChP volume and t-tau/p-tau for amyloid-positive and amyloid-negative groups separately, while we controlled for age, sex, APOEε4, cortical volume, and ventricular volume. To compare the predictive value of the ChP volume, cortical volume, and ventricular volume, we separately added each to a model that included age, sex, group, and APOEε4 (basic model) and measured adjusted R2. One-way ANOVA followed by the Tukey test to control for multiple comparisons was used to check for differences in the ChP volume between diagnostic groups. Python and R were used for the statistical analyses.
3. Results
3.1. Demographic information, CSF biomarkers, and imaging
Table 1 shows the demographic information (including age, gender, and APOEε4 status), levels of CSF biomarkers, brain volumes (including TBV, cortical volume, ventricular volume, and the ChP volume) for both the ADNI and PPMI data sets, and florbetapir (AV45) PET imaging SUVR values for the ADNI data set.
Table 1.
Demographic information, levels of CSF proteins, and imaging data in the ADNI and PPMI data sets
| Group | ADNI |
PPMI |
|||||
|---|---|---|---|---|---|---|---|
| Control | SMC | EMCI | LMCI | AD | Control | PD | |
|
| |||||||
| Demographic information | |||||||
| Number of subjects | 115 | 60 | 127 | 119 | 88 | 94 | 208 |
| Mean age | 73.4 ± 6.3 | 71.5 ± 5.4 | 71.3 ± 7.0 | 72.0 ± 7.8 | 74.6 ± 8.0 | 60.2 ± 11.3 | 61.4 ± 9.5 |
| Number of male (%) | 57 (49%) | 25 (41%) | 69 (54%) | 60 (50%) | 50 (56%) | 67 (71%) | 129 (69%) |
| APOE4 status (homozygous/heterozygous) | 6, 24 | 0, 19 | 6, 47 | 21, 38 | 20, 40 | 2, 23 | 5, 51 |
| CSF biomarkers (pg/mL) | |||||||
| t-tau | 241.9 ± 95.9 | 238.4 ± 91.7 | 254.3 ± 121.0 | 297.3 ± 130.0 | 379.3 ± 140.8 | 52.1 ± 29.9 | 44.1 ± 18.1 |
| p-tau | 22.2 ± 9.8 | 21.8 ± 9.8 | 24.1 ± 13.6 | 28.8 +/− 14.2 | 37.3 ± 14.9 | 18.9 ± 13.1 | 16.1 ± 9.5 |
| Aβ | 1404.2 ± 689.5 | 1358.5 ± 589.2 | 1176.9 ± 560.9 | 946.7 ± 483.4 | 717.2 ± 423.1 | 371.5 ± 93.1 | 375.2 ± 99.1 |
| α-syn | - | - | - | - | - | 2169.9 ± 1162.4 | 1855.4 ± 740.3 |
| Brain volumes (mL) | |||||||
| Total brain volume | 1504.8 ± 174.5 | 1516.1 ± 188.9 | 1516.3 ± 168.0 | 1529.3 ± 183.2 | 1557.0 ± 190.5 | 1528.4 ± 175.0 | 1586.1 ± 180.6 |
| Cortical volume | 435.4 ± 40.7 | 441.1 ± 42.5 | 436.5 ± 40.7 | 429.4 ± 39.9 | 409.5 ± 49.3 | 463.9 ± 45.4 | 468.8 ± 49.6 |
| Ventricular volume | 22.3 ± 10.4 | 20.3 ± 8.6 | 22.9 ± 11.6 | 26.6 ± 13.4 | 31.1 ± 12.7 | 28.6 ± 15.3 | 32.1 ± 20.2 |
| ChP volume (right + left) | 2.5 ± 0.6 | 2.5 ± 0.6 | 2.5 ± 0.6 | 2.6 ± 0.7 | 2.9 ± 0.5 | 2.4 ± 0.7 | 2.3 ± 0.7 |
| Left ChP volume | 1.3 ± 0.3 | 1.4 ± 0.4 | 1.4 ± 0.3 | 1.4 ± 0.4 | 1.6 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.4 |
| Right ChP volume | 1.2 ± 0.3 | 1.1 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.4 | 1.3 ± 0.2 | 1.1 ± 0.4 | 1.1 ± 0.3 |
| PET imaging | |||||||
| Florbetapir (AV45) (baseline) | 1.12 ± 0.19 | 1.13 ± 0.17 | 1.17 ± 0.20 | 1.26 ± 0.24 | 1.39 ± 0.21 | - | - |
| Amyloid positive (SUVR ≥ 1.11) (%) | 39 (33%) | 24 (40%) | 61 (48%) | 77 (64%) | 78 (88%) | - | - |
Key: ADNI, Alzheimer’s Disease Neuroimaging Initiative; ChP, choroid plexus; ChP, choroid plexus; APOEε4, apolipoprotein E ε4 allele; AD, Alzheimer’s disease; SMC, significant memory concern; EMCI, early mild cognitive impairment; LMCI, late mild cognitive impairment; t-tau, total-tau; p-tau, phosphorylated-tau; Aβ amyloid-β.
3.2. ChP segmentation
Fig. 1B shows the segmented ChP for 2 patients. There was a statistically significant difference in ChP volume between groups as determined by a one-way ANOVA test (F(6,776) = 7.98, p-value = 2.27×10−8).A Tukey post hoc test revealed that patients with AD had larger ChP volume compared with other diagnostic groups (p < 0.05).
3.3. Association between ChP volume and CSF proteins at the group level
Supplementary Table 1 provides results for univariate regression analyses to predict CSF proteins using age, sex, APOE ε4, diagnostic group, cortical volume, ventricular volume, and ChP volume as independent variables. ChP volume was negatively correlated with CSF proteins across both data sets (p < 0.01). In multivariate analyses, ChP volume showed significant association with t-tau (β = −0.33, p < 0.001) and p-tau (β = −0.34, p < 0.001) in ADNI-2, as well as t-tau (β = −0.24, p < 0.001), α-syn (β = −0.24, p < 0.001), and Aβ (β = −0.1, p < 0.001) in PPMI (Table 2). The associations between ChP volume and p-tau in PPMI and Aβ in ADNI-2 were not statistically significant (p = 0.09 and p = 0.03, respectively). VIF was less than 2 for all the tested models (no collinearity).
Table 2.
Multivariate analysis of CSF proteins in PPMI and ADNI-2
| Variables | t-tau |
p-tau |
Aβ |
α-syn |
||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | p | Coefficient | p | Coefficient | p | Coefficient | p | |
|
| ||||||||
| PPMI | ||||||||
| Age | 0.017 | <0.01 | 0.013 | <0.01 | 0.001 | - | 0.01 | <0.01 |
| Sex (male) | 0.01 | - | 0.05 | - | −0.01 | - | 0.12 | - |
| APOEε4 | 0.02 | - | 0.02 | - | −0.16 | <0.01 | −0.01 | - |
| ChP volume (mL) | −0.24 | <0.01 | −0.1 | - | −0.1 | <0.01 | −0.24 | <0.01 |
| Cortical volume (mL) | 0.51 | - | 1.09 | - | 0.79 | 0.01 | 0.3 | - |
| Ventricular volume (mL) | −0.002 | - | −0.006 | <0.01 | 0.001 | - | −0.002 | - |
| Group PD | −0.16 | <0.01 | - | - | - | - | −0.14 | <0.01 |
| ADNI | ||||||||
| Age | 0.015 | <0.01 | 0.17 | <0.01 | 0.004 | - | ||
| Sex (male) | −0.24 | - | −0.01 | - | −0.06 | - | ||
| APOEε4 | 0.2 | <0.01 | 0.24 | <0.01 | −0.42 | <0.01 | ||
| ChP volume (mL) | −0.33 | <0.01 | −0.34 | <0.01 | −0.176 | - | ||
| Cortical volume (mL) | 0.0008 | - | 0.0009 | - | 0.00124 | - | ||
| Ventricular volume (mL) | −0.004 | - | −0.004 | - | −0.007 | <0.01 | ||
| Group SMC | −0.009 | - | −0.01 | - | −0.01 | - | ||
| EMCI | 0.047 | - | 0.06 | - | −0.08 | - | ||
| LMCI | 0.197 | <0.01 | 0.23 | <0.01 | 0.23 | <0.01 | ||
| AD | 0.47 | <0.01 | 0.53 | <0.01 | −0.31 | <0.01 | ||
The coefficients (unstandardized) and p-values for each predictor.
Key: ADNI, Alzheimer’s Disease Neuroimaging Initiative; ChP, choroid plexus; APOEε4, apolipoprotein E ε4 allele; AD, Alzheimer’s disease; SMC, significant memory concern; EMCI, early mild cognitive impairment; LMCI, late mild cognitive impairment; t-tau, total-tau; p-tau, phosphorylated-tau; Aβ amyloid-β.
CSF proteins showed a positive correlation with each other in both the ADNI and PPMI data sets (Supplementary Figures 1 and 2). To capture the shared variance between CSF proteins, PCA was applied (Fig. 2A and D). The first principal component (PC1) explained around 60 percent of the variance in CSF proteins in both the ADNI and PPMI data sets (Fig. 2B and E). In PPMI, all CSF proteins had positive loadings in PC1 (i.e., all CSF proteins were positively correlated with each other). In ADNI, t-tau and p-tau showed the highest loadings in PC1, whereas Aβ had loading near zero. Fig. 2C and F show partial correlation of ChP volume and PC1 after controlling for age, sex, APOEε4 status, diagnostic group, and cortical volume (r partial = −0.43, p < 0.001 in PPMI and r partial =−0.40, p < 0.001 in ADNI-2).
Fig. 2.
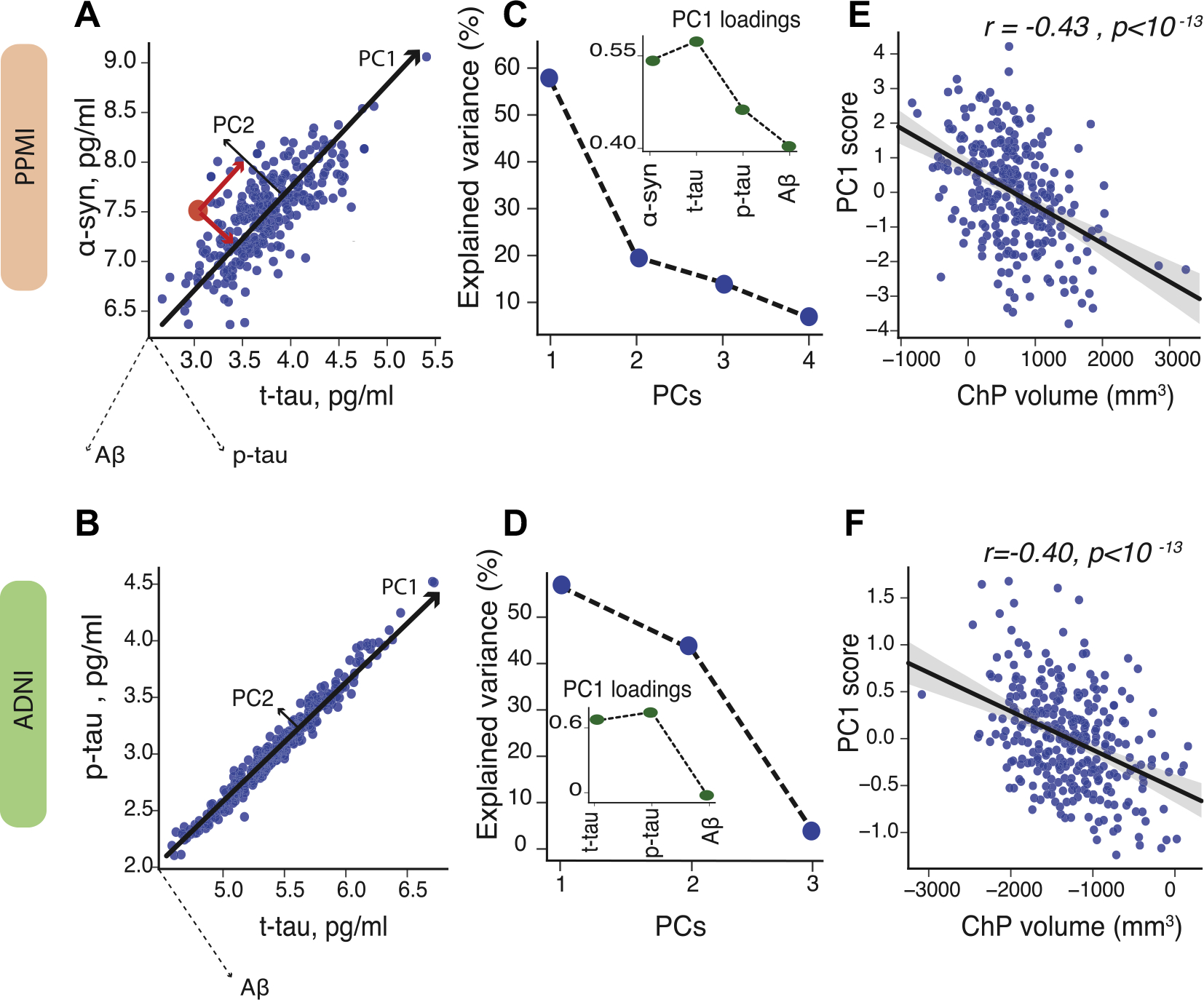
Principal component analysis (PCA) of CSF proteins. To capture the highest amount of variance in CSF proteins, we applied principal component analysis in PPMI (A) and ADNI-2 (D). PCA score is computed by projecting each data point coordinate (red dot) onto the corresponding principal components (red lines) (left plots; for illustration purposes only 2 CSF proteins are shown). The first principal component (PC1) explains around 60 percent of variance in CSF proteins in PPMI (B) and ADNI-2 (E). The insets show the loadings of PC1. The scatter plots show partial correlation between ChP volume and PC1, after correcting for age, sex, APOEε4 status, group, and cortical volume in PPMI (C) and ADNI (F). Note: levels of CSF proteins are log-tranformed. Abbreviations: ADNI, Alzheimer’s Disease Neuroimaging Initiative; ChP, choroid plexus; PC, principal component; PPMI, Parkinson’s Progression Markers Initiative. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. Association between ChP volume and CSF proteins for each diagnostic group
We examined the amount of variance explained by ChP volume in each diagnostic group separately. In ADNI, the amount of variance in CSF proteins explained by ChP volume was higher in healthy controls and EMCI compared with LMCI and AD (Fig. 3A), whereas the ChP volume explained a similar amount of variance in CSF proteins in healthy controls and patients with PD (Fig. 3B).
Fig. 3.
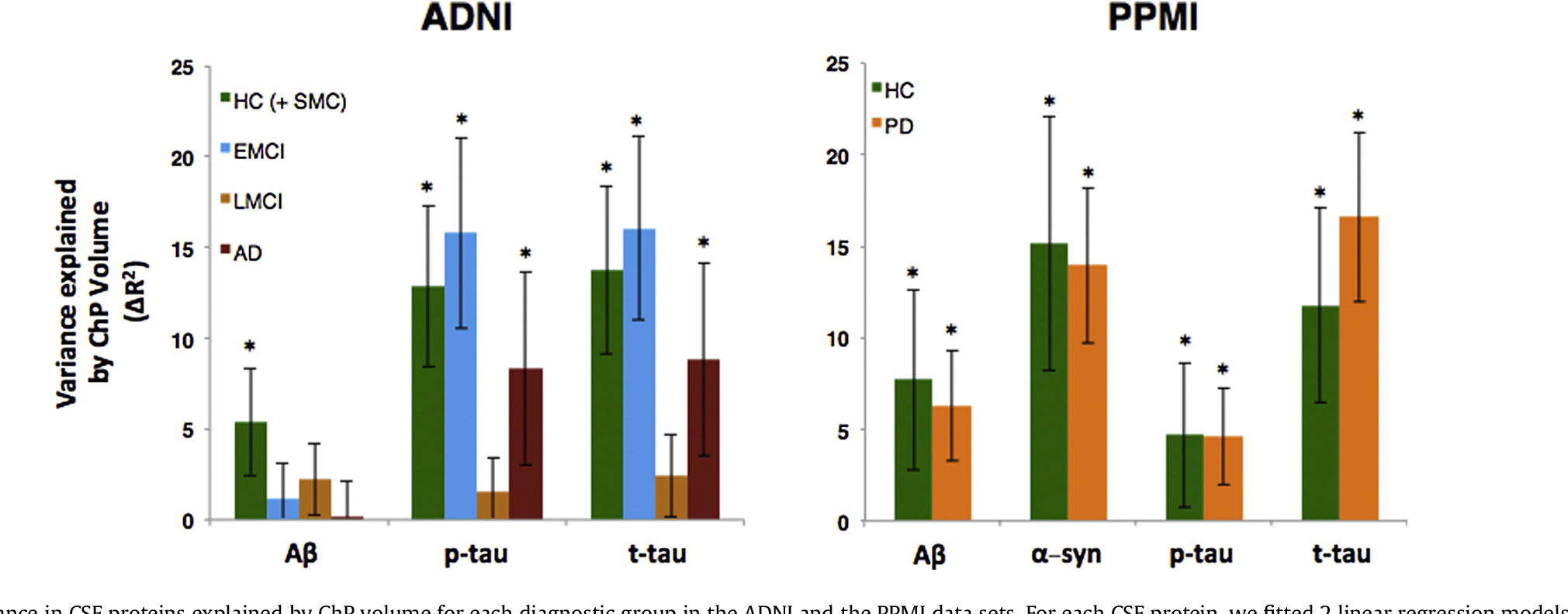
Variance in CSF proteins explained by ChP volume for each diagnostic group in the ADNI and the PPMI data sets. For each CSF protein, we fitted 2 linear regression models. The first model included age, sex, APOE ε4, cortical volume, and ChP volume as independent variables and the second model included age, sex, APOE ε4, and cortical volume as independent variables to predict the level of CSF protein. To measure the amount of variance explained by ChP volume, we then subtracted the adjusted R2 of the second model from the first model. The error bars indicate the standard deviation of change of R2 in 1000 bootstrapped samples. The 2 models were compared using F-test. The asterisk shows if the F-test was statistically significant (p < 0.01). Abbreviations: AD, Alzheimer’s disease; ADNI, Alzheimer’s Disease Neuroimaging Initiative; EMCI, early-mild cognitive impairment; HC, healthy controls; LMCI, late mild cognitive impairment; PD, Parkinson’s disease; PPMI, Parkinson’s Progression Markers Initiative; SMC, significant memory concern
3.5. Florbetapir amyloid-positive patients have lower association between the ChP volume and t-tau/p-tau
In ADNI, participants were grouped into amyloid-positive and amyloid-negative based on their florbetapir (AV45) PET imaging SUVR (AV45 ≥ 1.11) (Table 1). The multiple linear regression models showed a trend that the association between ChP volume and CSF proteins was lower in amyloid-positive group (for t-tau: βChP = −0.32, p-value < 0.001 and βAβ+ × ChP = 0.09, p-value = 0.07; for p-tau: βChP = −0.25, p-value < 0.001 and βAβ + × ChP = 0.12, p-value = 0.04). Importantly, the interaction term (βAβ+ × ChP) was positive, which indicates that the negative association between ChP volume and CSF proteins (βChP) is less in amyloid-positive patients. Fig. 4 shows the partial correlation between ChP volume and t-tau/p-tau in amyloid-positive and amyloid-negative groups, controlled for the confounding variables.
Fig. 4.
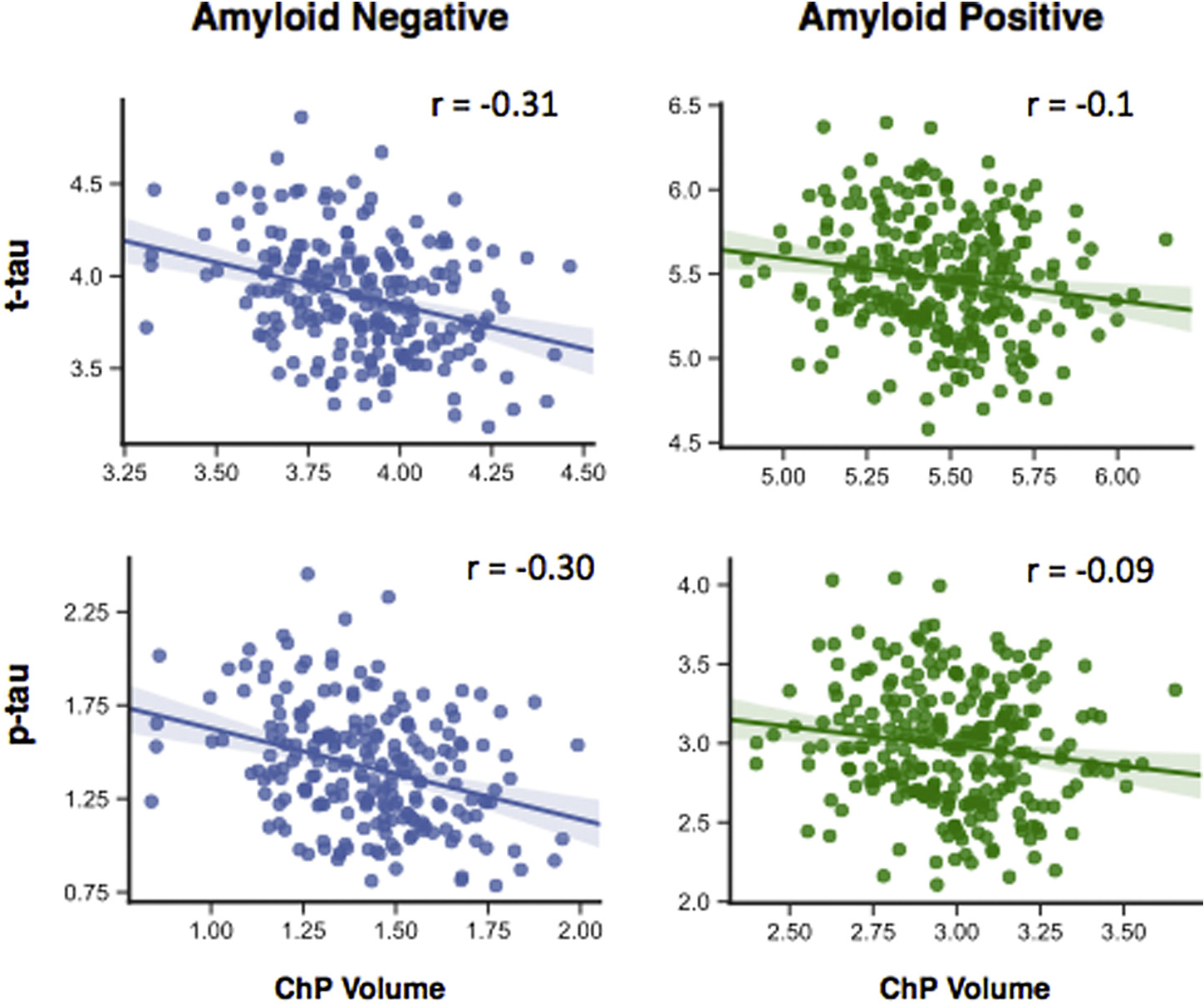
Partial correlation between ChP volume and t-tau and p-tau in the amyloid-positive and amyloid-negative groups in ADNI dataset. Participants were divided into amyloid-positive based on the AV45 SUVR at the baseline (AV45 ≥ 1.11) Abbreviation: ChP, choroid plexus. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.6. Cortical volume, ventricular volume, and CSF proteins
Cortical volume did not show any significant association with CSF proteins in analyses of PPMI data, whereas it was negatively associated with t-tau/p-tau and positively associated with Aβ in ADNI-2 cohort (Supplementary Table 1). Multivariate analyses only showed a significant association between cortical volume and Aβ in PPMI (Table 2). In univariate regression analysis, ventricular volume showed negative associations with t-tau, α-syn, p-tau, and Aβ in PPMI and only with Aβ in ADNI-2 (Supplementary Table 1). In multivariate regression analyses, the associations between CSF proteins and ventricular volume were not statistically significant except for p-tau in PPMI and Aβ in ADNI-2 (Table 2).
Cortical volume showed a weak correlation with ChP volume in PPMI (r = 0.12, p < 0.05) and ADNI-2 (r = 0.16, p < 0.001), whereas ventricular volume was positively correlated with the ChP volume across the 2 cohorts (r ~ 0.63, p < 10−3) (Fig. 5B). To compare the predictive value of cortical, ventricular, and ChP volume, we separately added each predictor to a model including age, sex, APOEε4 status, and diagnostic group to predict CSF proteins (basic model) (Fig. 5C). The model that included ChP volume showed higher adjusted R2 compared with the models that included cortical volume or ventricular volume for Aβ, p-tau, and t-tau in the ADNI and Aβ, t-tau, and α-syn in PPMI.
Fig. 5.
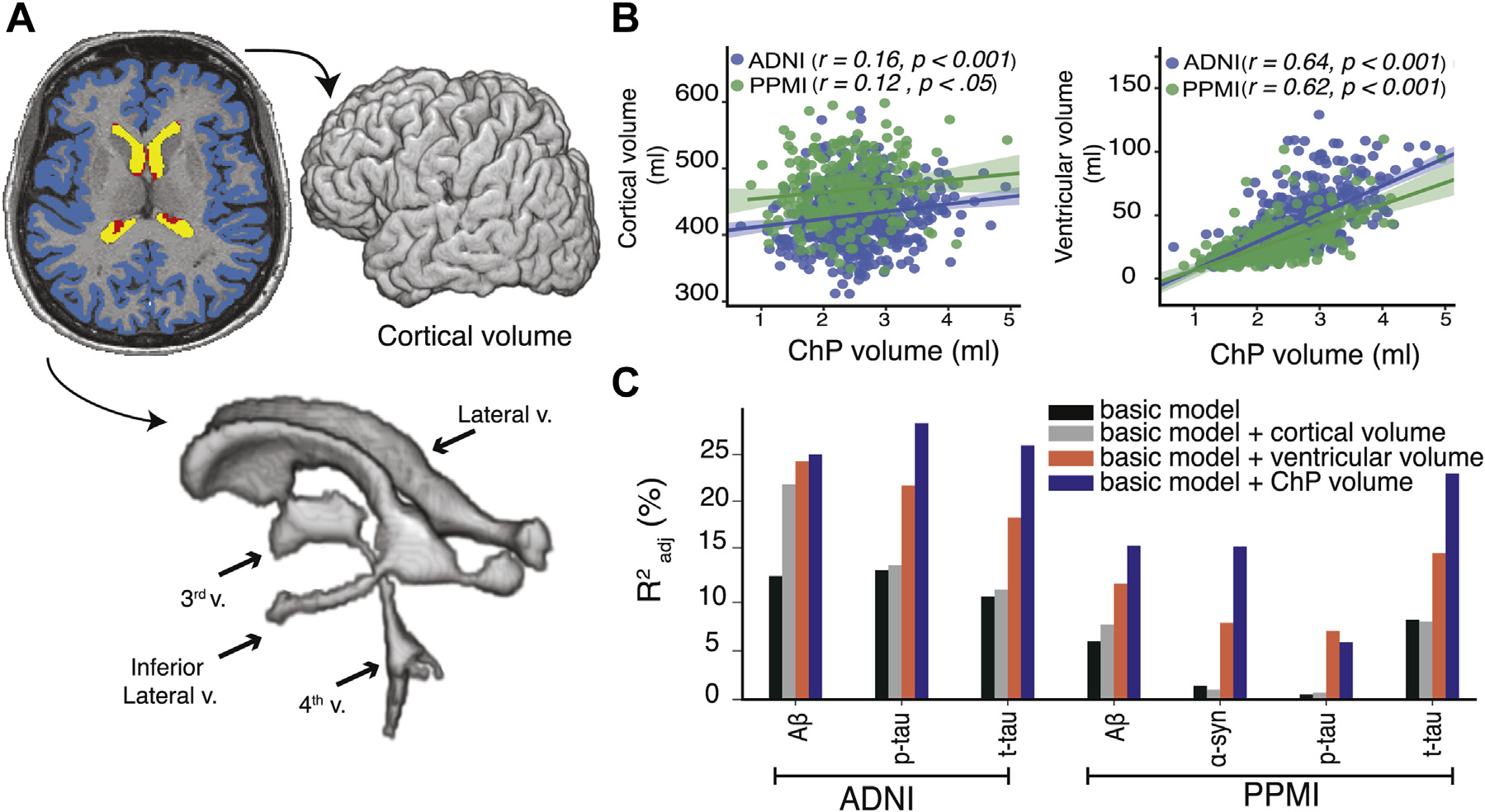
Cortical volume, ventricular volume, and their associations with CSF proteins compared with ChP volume. Cortical volume was measured by summing volume of left and right cortices (blue). Ventricular volume was measured by summing volumes of lateral ventricles (yellow), inferior lateral ventricle horns, third and fourth ventricle (ChP segmentation is in red) (A). ChP volume was correlated with cortical volume (r ~ 0.14) and ventricular volume (r ~ 0.63) (B). ChP volume, ventricular volume, and cortical volume were separately added to the basic model, which included age, sex, diagnostic group and APOEε4 status (C). Abbreviations: ADNI, Alzheimer’s Disease Neuroimaging Initiative; ChP, choroid plexus; PPMI, Parkinson’s Progression Markers Initiative. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
We examined the association between the ChP volume and CSF proteins in a large cohort of healthy controls, SMC patients, EMCI patients, LMCI patients, and AD patients (ADNI cohort), and healthy controls and PD patients (PPMI cohort). We found a negative association between the ChP volume and levels of each tested CSF protein in both univariate and multivariate regression analyses at the group level. To account for the positive correlation between CSF proteins, we applied PCA to capture the shared variance in CSF proteins and found that the ChP volume had a negative association with the first principal component (PC1). We then showed that the association between the ChP volume and levels of CSF proteins was highest in healthy controls and EMCI patients, with a lower association in LMCI and AD groups in the ADNI cohort. On the other hand, ChP volume showed similar association with levels of CSF proteins in healthy controls and patients with PD in the PPMI cohort. Using florbetapir PET imaging data, we grouped participants into amyloid-positive and amyloid-negative and found that the association between ChP volume and t-tau/p-tau was lower in amyloid-positive group. We finally demonstrated that ChP volume had a stronger association with CSF proteins than ventricular volume and cortical volume. Our results are in line with prior animal and in vitro studies that suggest a possible role of ChP in the clearance of CSF proteins, support possible ChP dysfunction in AD, and highlight ChP volume as a contributing factor to interindividual variance in levels of CSF proteins.
We hypothesized that larger ChP volume would be associated with lower levels of CSF proteins for 2 reasons: (i) indirectly through higher production of CSF proteins and (ii) directly by providing larger number of CSF-blood protein transporters on the ChP epithelial tissue. Although no study has been performed to show the relationship between ChP volume and the amount of CSF secretion, there are lines of evidence in favor of this hypothesis. For instance, choroid plexus papillomas are benign congenital intracranial tumors that are mostly found in the lateral ventricles and one of its major complications is hydrocephalus. One major mechanism behind choroid plexus papillomas’ hydrocephalus is overproductions of CSF from the choroid plexus tissue (Pawar et al., 2003; Sachan, 2017). Thus, it can indirectly be inferred that larger ChP volume can lead to increased CSF production through increased number of functional units. Increased CSF production can enhance the activity of CSF-dependent brain clearance systems. For instance, the newly described glymphatic pathway starts with CSF entry into the brain via periarterial spaces with entrance into interstitium via aquaporin-4 water channels expressed on astrocytes (Jessen et al., 2015). This creates a convective current that drives interstitial fluid into perivenous spaces and clear brain of waste products such as Aβ (Iliff et al., 2012). Once inside the CSF, waste products are absorbed into the circulatory or lymphatic systems.
Furthermore, recent molecular studies have attributed new functionalities to ChP, highlighting its direct role in clearing CSF proteins. To that end, 2 conditions should be met: (i) proteins produced in the brain should be able to enter ventricles adjacent to choroid plexus and (ii) choroid plexus should be able to capture these proteins. As for the former, studies using horseradish peroxidase and fluorescent tracers injected into brain parenchyma have shown entrance of tracers into lateral ventricles mainly via interstitial fluid bulk flow after passing through ependymal layer (Bedussi et al., 2015; Cserr et al., 1977), supporting the idea that brain-derived proteins can enter lateral ventricles adjacent to ChP. Moreover, molecular studies of ChP epithelial cells have revealed many transporters on the apical side of the epithelium responsible for transporting proteins. For instance, many of the Aβ transporters (such as LRP1, LRP2, RAGE, AΒCB1) that are normally expressed at the blood-brain barrier have been also localized on ChP epithelium on the CSF side (Crossgrove et al., 2005). One study showed that radiolabeled Aβ ([125I]hAβ(1–40)) injected into rat’s lateral ventricles are taken up by ChP and removed from CSF 5 times faster if it was only removed via CSF bulk flow, further emphasizing the direct role of ChP in clearing CSF proteins (Fujiyoshi et al., 2011).
Previous animal studies have shown pathological changes of ChP in AD, possibly leading to ChP dysfunction (Balusu et al., 2016; Krzyzanowska and Carro, 2012). This has led some groups to test the therapeutic role of ChP epithelial stem cells in AD treatment with promising results (Aliaghaei et al., 2015; Bolos et al., 2014). In line with animal studies, we found dissociation between the amount of CSF proteins explained by ChP volume across AD spectrum disorders, with a stronger association in healthy controls and EMCI groups compared with LMCI and AD patients. CSF proteins in healthy controls and patients with PD showed more or less similar association with ChP volume, possibly suggesting a disease-specific role of ChP in AD. As Aβ accumulates early in the disease (before any apparent cognitive changes or changes in t-tau and p-tau levels) (Jack and Holtzman, 2013), we furthered our analyses by grouping the ADNI subjects into amyloid-positive and -negative based on their florbetapir PET imaging data. We found that amyloid-positive patients had lower association between ChP volume and t-tau and p-tau than amyloid-negative patients. However, future studies with larger sample sizes and robust imaging techniques for measuring CSF production and clearance dysfunction are needed to causally evaluate if ChP dysfunction precedes Aβ accumulation.
Prior studies have suggested a possible association between brain volumetric measurements and CSF proteins; however, these studies have mainly focused on ventricular volume while largely ignoring ChP volume as a possible factor explaining the interindividual difference of CSF proteins. In a study of healthy controls and patients with AD, Ott et al. (Ott et al., 2010) reported a negative association between ventricular volume and CSF t-tau in AD patients. More recently, in a study of 730 healthy controls, MCI patients, and AD patients, a negative association was found between ventricular volume and Aβ, while the association for t-tau and p-tau was not significant (van Waalwijk van Doorn et al., 2017). In another recent study, ventricular volume showed a negative association with Aβ–38 and Aβ–40 with no significant association with t-tau, p-tau, and Aβ–42 (Edsbagge et al., 2017). Two main hypotheses can explain the possible association between ventricular volume and CSF proteins. The first hypothesis argues that disease severity can explain the negative association between Aβ and ventricular volume as CSF Aβ declines while ventricular volume increases with disease severity. Based on this hypothesis, one would expect to find a positive correlation between ventricular volume and t-tau/p-tau, as CSF concentrations of these proteins increase with disease severity (Sämgård et al., 2010). However, previous studies have either found negative associations between ventricular volume and t-tau and p-tau, or no association at all. The second hypothesis argues for the dilutional effect of increased ventricular volume, predicting a negative correlation between various CSF proteins and ventricular volume. Although the dilutional effect of ventricular volume cannot be rejected, our results show a stronger association between CSF proteins/ChP volume compared with CSF proteins/ventricular volume. Given that the ChP volume and ventricular volume show a significant correlation, previous reported associations between ventricular volume and CSF proteins, albeit weak, could be explained—and reinterpreted—by ChP volume.
Finally, levels of most CSF proteins are positively correlated with each other. The association between t-tau and Aβ is important to note as previous studies have shown a complex and nonlinear dynamic association between the 2 in AD (de Leon et al., 2018). This can raise the question how the ChP volume can be negatively correlated with both t-tau and Aβ. To answer this concern, we showed that Aβ and t-tau had positive association in both healthy controls and patients with PD in PPMI, as well as healthy controls of the ADNI (Supplementary Figures 1 and 2). The direction of the association changed to negative in the EMCI and the LMCI groups, with no association in the AD group. This confirmed the dynamic nature of correlation between Aβ and t-tau in the EMCI/LMCI/AD groups. To account for the correlated variation between CSF proteins, we performed PCA. We were able to show that ChP volume is associated with the first principal component (PC1), which explained about 60% of the variance in levels of CSF proteins. Together, these suggest that ChP volume is a contributing factor to all CSF proteins, possibly accounting for the shared variance between them.
5. Study limitations and future directions
In the present study, we indirectly measured the volume of ChP from structural T1-weighted MRIs. The gold-standard technique to noninvasively visualize ChP is T1-weighted MRIs enhanced with contrast. The fenestrated endothelium in ChP allows contrast to accumulate in the interstitium, whereas the ChP-CSF barrier precludes contrast to leak into the CSF (Shi et al., 2017). However, contrast-enhancing agents are not used in research studies routinely and their usage is mainly limited to clinical settings where the benefits outweigh their risks. On the other hand, high-resolution T1-w MRIs are routinely acquired in research studies, where ChP has intensity similar to gray matter voxels. T1-weighted MRIs can be utilized to study ChP within lateral ventricles, which have the largest ChP among all brain ventricles. However, they do not have enough resolution for segmenting ChP within third and fourth ventricles. New MRI sequences, as well as 7T anatomical MRIs, could provide better resolution to study ChP within third and fourth ventricles without the need to use contrast-enhancing agents.
Although ChP within lateral ventricles can be manually segmented, it precludes large-scale neuroimaging studies given its time-consuming nature. This highlights the need for accurate automatic ChP segmentation techniques. Freesurfer software has been used in most previous studies for automatic ChP segmentation; however, future studies are needed to accurately measure its accuracy. Moreover, new algorithms that are developed solely to segment ChP could be beneficial in studying the structure and function in large-scale neuroimaging studies. It is important to note that ChP can undergo calcification that might affect the segmentation accuracy. Although generally considered benign, future studies are needed to study if ChP calcification can impact ChP volumetric measurements.
We found that the ChP volume was larger in AD compared with other groups. Although the difference in the ChP volume was statistically significant between AD and other groups, the effect size is minimal. Several factors could explain the observed larger ChP volume in AD. Given that ventricles are enlarged in patients with AD, this could lead to the overestimation of ChP segmentation due to automatic segmentation inaccuracy. Reduced pressure in ventricles as a result of ventricular enlargement can also lead to apparent ChP enlargement in AD.
Aside from measuring ChP volume, new techniques can be used to obtain information About the structural and functional properties of ChP. MRI techniques such as arterial spin labeling (ASL) might provide important information regarding ChP perfusion, given its highly vascular stroma and substantial blood perfusion relative to the brain parenchyma (Dangouloff-Ros et al., 2015). In addition, developing new techniques that allow noninvasive measurement of CSF production rate as well as assessing glymphatic system efficiency can open new windows into identifying patients at risk of developing AD (Harrison et al., 2018).
6. Conclusion
We found a negative association between ChP volume and levels of CSF proteins. Moreover, we showed that the association between ChP volume and CSF proteins is low in LMCI and AD groups compared with healthy controls, EMCI, and PD patients. Our results support a potential role of ChP in CSF protein clearance and corroborate prior animal studies showing ChP dysfunction in AD. Our results also introduce ChP volume as a contributing factor to the variability in levels of CSF proteins across subjects and suggest the importance of accounting for ChP volume in future studies of CSF-based biomarkers.
Supplementary Material
Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2–0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
ES and APL are supported by the BROAD Institute at Harvard-MIT (Boston, MA) via 2016P000351. ES and APL are partially supported by Defense Advanced Research Projects Agency (DARPA) via HR001117S0030. ES is supported by the Beth Israel Deaconess Medical Center (BIDMC) via the Chief Academic Officer (CAO) grant 2017. APL is further supported by the Berenson-Allen Foundation, the Sidney R. Baer Jr. Foundation, grants from the National Institutes of Health (R01HD069776, R01NS073601, R21 MH099196, R21 NS082870, R21 NS085491, R21 HD07616), and Harvard Catalyst, The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health, the Sidney R. Baer Jr. Foundation.
Footnotes
Disclosure statement
All authors report no conflict of interest.
CRediT authorship contribution statement
Ehsan Tadayon: Conceptualization, Methodology, Software, Formal analysis, Writing - original draft, Writing - review & editing, Visualization. Alvaro Pascual-Leone: Writing - original draft, Supervision. Daniel Press: Writing - review & editing. Emiliano Santarnecchi: Writing - original draft, Writing - review & editing, Supervision.
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wpcontent/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neurobiolaging.2020.01.005.
References
- Aliaghaei A, Digaleh H, Khodagholi F, Ahmadiani A, 2015. Encapsulated choroid plexus epithelial cells actively protect against intrahippocampal Aβ-induced long-term memory dysfunction; upregulation of effective neurogenesis with the abrogated apoptosis and neuroinflammation. J. Mol. Neurosci 56, 708–721. [DOI] [PubMed] [Google Scholar]
- Alvira-Botero X, Carro EM, 2010. Clearance of amyloid-β peptide across the choroid plexus in Alzheimer’s disease. Curr. Aging Sci 3, 219–229. [DOI] [PubMed] [Google Scholar]
- Andersen AD, Binzer M, Stenager E, Gramsbergen JB, 2017. Cerebrospinal fluid biomarkers for Parkinson’s disease – a systematic review. Acta Neurol. Scand 135, 34–56. [DOI] [PubMed] [Google Scholar]
- Baker SL, Maass A, Jagust WJ, 2017. Considerations and code for partial volume correcting [18F]-AV-1451 tau PET data. Data Brief 15, 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balusu S, Brkic M, Libert C, Vandenbroucke RE, 2016. The choroid plexus-cerebrospinal fluid interface in Alzheimer’s disease: more than just a barrier. Neural Regen. Res 11, 534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedussi B, van Lier MG, Bartstra JW, de Vos J, Siebes M, VanBavel E, Bakker EN, 2015. Clearance from the mouse brain by convection of interstitial fluid towards the ventricular system. Fluids Barriers CNS 12, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE, 2016. Choroid plexus–CSF system: recent developments and clinical correlations. Neurology 86, 286–296. [DOI] [PubMed] [Google Scholar]
- Blennow K, Hampel H, Weiner M, Zetterberg H, 2010. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol 6, 131–144. [DOI] [PubMed] [Google Scholar]
- Bolos M, Antequera D, Aldudo J, Kristen H, Bullido MJ, Carro E, 2014. Choroid plexus implants rescue Alzheimer’s disease-like pathologies by modulating amyloid-b degradation. Cell. Mol. Life Sci 71, 2947–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossgrove JS, Li GJ, Zheng W, 2005. The choroid plexus removes beta-amyloid from brain cerebrospinal fluid. Exp. Biol. Med. (Maywood) 230, 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserr HF, Cooper DN, Milhorat TH, 1977. Flow of cerebral interstitial fluid as indicated by the removal of extracellular markers from rat caudate nucleus. Exp. Eye Res 25, 461–473. [DOI] [PubMed] [Google Scholar]
- Damkier HH, Brown PD, Praetorius J, 2013. Cerebrospinal fluid secretion by the choroid plexus. Physiol. Rev 93, 1847–1892. [DOI] [PubMed] [Google Scholar]
- Dangouloff-Ros V, Grevent D, Pagès M, Blauwblomme T, Calmon R, Elie C, Puget S, Sainte-Rose C, Brunelle F, Varlet P, Boddaert N, 2015. Choroid plexus neoplasms: toward a distinction between carcinoma and papilloma using arterial spin-labeling. AJNR Am. J. Neuroradiol 36, 1786–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon MJ, Pirraglia E, Osorio RS, Glodzik L, Saint-Louis L, Kim H-J, Fortea J, Fossati S, Laska E, Siegel C, Butler T, Li Y, Rusinek H, Zetterberg H, Blennow K, Alzheimer’s Disease Neuroimaging Initiative, National Alzheimer’s Coordinating Center, 2018. The nonlinear relationship between cerebrospinal fluid Aβ42 and tau in preclinical Alzheimer’s disease. PLoS One 13, e0191240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsbagge M, Andreasson U, Ambarki K, Wikkelsø C, Eklund A, Blennow K, Zetterberg H, Tullberg M, 2017. Alzheimer’s disease-associated cerebrospinal fluid (CSF) biomarkers do not correlate with CSF volumes or CSF production rate. J. Alzheimers Dis 58, 821–828. 10.3233/JAD-161257. [DOI] [PubMed] [Google Scholar]
- Fujiyoshi M, Tachikawa M, Ohtsuki S, Ito S, Uchida Y, Akanuma S-I, Kamiie J, Hashimoto T, Hosoya K-I, Iwatsubo T, Terasaki T, 2011. Amyloid-β peptide(1–40) elimination from cerebrospinal fluid involves low-density lipoprotein receptor-related protein 1 at the blood-cerebrospinal fluid barrier. J. Neurochem 118, 407–415. [DOI] [PubMed] [Google Scholar]
- Harrison IF, Siow B, Akilo AB, Evans PG, Ismail O, Ohene Y, Nahavandi P, Thomas DL, Lythgoe MF, Wells JA, 2018. Non-invasive imaging of CSF-mediated brain clearance pathways via assessment of perivascular fluid movement with diffusion tensor MRI. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Leverenz JB, Baird G, Montine TJ, Hancock AM, Hwang H, Pan C, Bradner J, Kang UJ, Jensen PH, Zhang J, 2010. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain 133, 713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M, 2012. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med 4, 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Holtzman DM, 2013. Biomarker modeling of Alzheimer’s disease. Neuron 80, 1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen NA, Munk ASF, Lundgaard I, Nedergaard M, 2015. The glymphatic system: a beginner’s guide. Neurochem. Res 40, 2583–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzanowska A, Carro E, 2012. Pathological alteration in the choroid plexus of Alzheimer’s disease: implication for new therapy approaches. Front. Pharmacol 3, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Lu M, Joshi AD, Pontecorvo M, Mintun MA, Trojanowski JQ, Shaw LM, Jagust WJ, Alzheimer’s Disease Neuroimaging Initiative, 2013. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann. Neurol 74, 826–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ, 2010. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 330, 1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott BR, Cohen RA, Gongvatana A, Okonkwo OC, Johanson CE, Stopa EG, Donahue JE, Silverberg GD, Alzheimer’s Disease Neuroimaging Initiative, 2010. Brain ventricular volume and cerebrospinal fluid biomarkers of Alzheimer’s disease. J. Alzheimers Dis 20, 647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl DP, 2010. Neuropathology of Alzheimer’s disease. Mt. Sinai J. Med 77, 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar SJ, Sharma RR, Mahapatra AK, Lad SD, Musa MM, 2003. Choroid plexus papilloma of the posterior third ventricle during infancy & childhood: report of two cases with management morbidities. Neurol. India 51, 379–382. [PubMed] [Google Scholar]
- Sachan D, 2017. Choroid plexus papilloma causing CSF shunt ascites: A Rare Presentation. Ann Clin Case Rep 2, 1376. [Google Scholar]
- Sämgård K, Zetterberg H, Blennow K, Hansson O, Minthon L, Londos E, 2010. Cerebrospinal fluid total tau as a marker of Alzheimer’s disease intensity. Int. J. Geriatr. Psychiatry 25, 403–410. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Landau SM, Fero A, Schreiber F, Jagust WJ, 2015. Alzheimer’s Disease Neuroimaging Initiative, 2015. Comparison of Visual and Quantitative Florbetapir F 18 Positron Emission Tomography Analysis in Predicting Mild Cognitive Impairment Outcomes. JAMA Neurol 72, 1183–1190. [DOI] [PubMed] [Google Scholar]
- Shi Y, Li X, Chen X, Xu Y, Bo G, Zhou H, Liu Y, Zhou G, Wang Z, 2017. Imaging findings of extraventricular choroid plexus papillomas: a study of 10 cases. Oncol. Lett 13, 1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ, 2015. Clearance systems in the brain—implications for Alzheimer disease. Nat. Rev. Neurol 11, 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Waalwijk van Doorn LJC, Gispert JD, Kuiperij HB, Claassen JAHR, Arighi A, Baldeiras I, Blennow K, Bozzali M, Castelo-Branco M, Cavedo E, Emek-Savaş DD, Eren E, Eusebi P, Farotti L, Fenoglio C, Ormaechea JF, Freund-Levi Y, Frisoni GB, Galimberti D, Genc S, Greco V, Hampel H, Herukka S-K, Liu Y, Lladó A, Lleó A, Nobili FM, Oguz KK, Parnetti L, Pereira J, Picco A, Pikkarainen M, de Oliveira CR, Saka E, Salvadori N, Sanchez-Valle R, Santana I, Scarpini E, Scheltens P, Soininen H, Tarducci R, Teunissen C, Tsolaki M, Urbani A, Vilaplana E, Visser PJ, Wallin AK, Yener G, Molinuevo JL, Meulenbroek O, Verbeek MM, 2017. Improved cerebrospinal fluid-based discrimination between Alzheimer’s disease patients and controls after correction for ventricular volumes. J. Alzheimers Dis 56, 543–555. [DOI] [PubMed] [Google Scholar]
- Zhou G, Hotta J, Lehtinen MK, Forss N, Hari R, 2015. Enlargement of choroid plexus in complex regional pain syndrome. Sci. Rep 5, 14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


