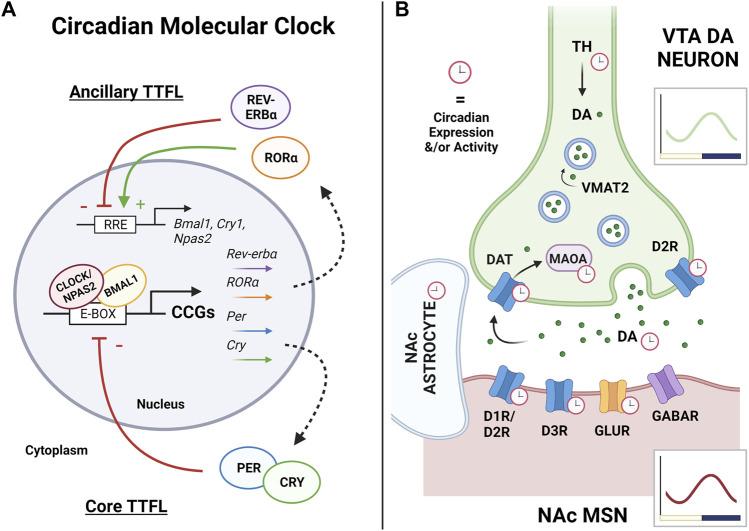
FIGURE 1.
The circadian molecular clock and its regulation of the VTA-NAc synapse. (A) Rhythms of the circadian molecular clock are generated through a complex series of transcriptional-translational feedback loops (TTFLs). In the mammalian core TTFL, CLOCK (or its functional paralogue NPAS2) forms a heterodimer with BMAL1, binds to E-box elements in the promoter regions of DNA, and drives the transcription of many clock-controlled genes (CCGs), including Period and Cryptochrome. Throughout the day, PERs and CRYs accumulate in the cytoplasm, dimerize, and undergo phosphorylation. Into the night, PER:CRY dimers shuttle back into the nucleus and inhibit their own transcription through repressing CLOCK/NPAS2:BMAL1 activity, thus completing the core TTFL which cycles approximately every 24 h. Among its CCGs, CLOCK/NPAS2:BMAL1 also regulates the expression of the nuclear receptors RORα and Rev-erbα which form an ancillary TTFL of the molecular clock. RORα and REV-ERBα compete at Rev-erb/Ror response elements (RREs) in the promoter regions of Bmal1, Npas2, and Cry1 to regulate their transcription, where RORα promotes expression and REV-ERBα represses. Along with others, this ancillary TTFL works to sustain, stabilize, and reinforce the core TTFL and rhythmic output as a whole, altogether temporally controlling nearly all aspects of cellular physiology. Arrows and (+) indicate promotion of expression, while bars and (−) indicate repression of expression. (B) The ventral tegmental area (VTA) and nucleus accumbens (NAc) circuit is the key reward pathway of the brain. Rewarding stimuli primarily stimulate dopamine (DA) neurons in the VTA to release DA into the NAc, driving medium spiny neuron (MSN) activity and subsequent reward-seeking behaviors. The circadian molecular clock has been shown to regulate nearly all components of DA synaptic transmission, with synthesis, uptake, and degradation all showing circadian variation in expression or activity. This includes the synthesis enzyme tyrosine hydroxylase (TH), the DA receptors (D1R, D2R, and D3R), DA release itself, the dopamine reuptake transporter (DAT), and the dopamine degradation enzyme monoamine oxidase A (MAOA). Overall rhythms in activity, molecular clock genes, and transcriptome wide rhythms have been detected in the VTA and NAc, with peaks aligning in the active phase (dark phase in nocturnal rodents). Robust rhythms have been detected in NAc astrocytes, as well as rhythms in GABA, glutamate, and glutamate receptors (GLUR) in the NAc. Red clocks indicate observed rhythms in expression or activity. Green dots are DA molecules. Vesicular monoamine transporter 2 (VMAT2) loads dopamine into the vesicles prior to synaptic release. Graphs illustrate representative wave form of overall VTA (green) or NAc (red) rhythmicity in nocturnal rodents, with yellow bar indicating light phase and purple bar indicating dark phase. Figure created with BioRender.com.