Abstract
Background
The novel coronavirus disease, commonly called COVID-19, has already killed millions of lives. Our study aimed to identify a safe and right drug for the management of such globally threatened COVID-19.
Methods
This preliminary double-blinded randomized controlled trial was done among 57 hospitalized COVID-19 patients in the early stage of their illness. Of them, 29 patients received Favipiravir (FVP) and the remaining 28 patients received a placebo under the standard of care. Among the patients, 4 from Favipiravir (FVP) group and 3 from the placebo group were discontinued. The patients were observed regularly for a period of 10 days.
Result
In our study, the FVP treated group showed accelerated viral clearance compared to the placebo-treated group. Assessment of chest X-ray showed remarkable improvement of pheumonia patient in group A compared to Group B. Hematological and Biochemical parameters such as total WBC count, neutrophil and lymphocyte counts were examined. No significant differences in the hematological parameters such as WBC count, neutrophil and lymphocyte counts in Group A and Group B patients. Liver transaminases levels were also stable in FVP treated group (average ALT ranges 39.4–46.2; AST 28.2–32.8).
Conclusion
The drug Favipiravir displayed remarkable improvements in the clinical conditions and recovery of COVID-19 patients at the early stages of their infections.
Keywords: Bangladesh, COVID-19, Favipiravir, Infectious disease, SARS-CoV-2
Abbreviations: FVP, Favipiravir
Introduction
A recent outbreak of a highly contagious viral disease called COVID-19 was caused by the novel coronavirus. This modified form of coronavirus was first detected in Wuhan, China at the end of 2019, and was labeled as severe acute respiratory syndrome coronavirus 2 (SARS CoV 2) (Lu et al., 2020). The clinical features of COVID-19 include respiratory symptoms (cough, dyspnea, phlegm, runny nose, chest tightness), fever, headache, anosmia, fatigue, diarrhea, nausea, vomiting, and pneumonia (Wang et al., 2020, Huang et al., 2020). Some patients also noticed septic shock, acute respiratory distress symptoms (ARDS), metabolic dysfunctions, and coagulopathy in the severe stage (Zhou et al., 2020, Weiss and Murdoch, 2020). By Worldometer (2021) more than 132 million infected cases were reported, and about 2.8 million deaths were recorded across the world (Worldometer, 2021). On March 11, 2020, the World Health Organization (WHO) declared COVID-19 a pandemic and a worldwide public health emergency due to its rapid transmission and severity of symptoms including a higher risk of mortality (Cucinotta and Vanelli, 2020). This outbreak has also adversely affected education, economy, food security, and other activities globally, posing a threat to achieving the sustainable development goals (SDGs) (Hossain et al., 2021, Nicola et al., 2020, Leal Filho et al., 2020).
Considering this catastrophe, stopping the spread of the disease and treating the affected people has become an indispensable task to save lives and resume usual life. Scientists predict that an effective and safe vaccine or drugs can exterminate this invisible enemy and restore a normal lifestyle in the world. However, vaccine or drug development is a lengthy process that needs continuous efforts for several years. Therefore, it is urgently warranted to identify any existing effective antiviral agents that can combat this virus. Many researchers recommend that exploration of drugs through observing clinical effects can be a wise attempt to identify the right drugs against COVID-19 (Hossain and Rahman, 2021, Alam et al., 2021). Several antiviral therapies such as Remdisivir (RSV), Interferon (IFN), Favipiravir (FPV), and Lopinavir (LPV)/ritonavir (RTV) are already being investigated against SARS-CoV-2 and several clinical trials are underway in different countries (Li and Clercq, 2020, NIH US, 2020).
Favipiravir is an antiviral prodrug developed by the Japanese company “Fujifilm Toyama Chemical” usually used against the influenza virus (Furuta et al., 2013). It was also applied for the treatment of Ebola and other RNA viruses (Nagata et al., 2015, Delang et al., 2018, Sleeman et al., 2010). Interestingly, it was observed that, FPV can effectively inhibit the SARS-CoV-2 infection in Vero E6 cells (half-maximal effective concentration (EC50) = 61.88 μmol, half-maximal cytotoxic concentration (CC50) > 400 μmol, and selectivity index (SI) > 6.46) (Wang et al., 2020). Moreover, in an open-label study in China against mild to moderate COVID-19 patients, FPV along with Interferon-a (IFN-a) was found to accelerate the viral clearance compared to lopinavir (LPV), ritonavir (RTV), and IFN-a in the control arm (Cai et al., 2020).
One of the studies showed that around 80% of COVID-19 patients faced mild to moderate acute respiratory distress syndrome (ARDS) (Roser et al., 2020). A study depicts that due to an extensive viral replication among co-morbid patients, they developed severe ARDS, thus increasing the risk of death (Callender et al., 2020). However, to the best of our knowledge, there has been no specific clinical trial on FPV in Bangladesh. Hence, the authors felt the necessity to conduct a study on FPV to see the efficacy and effectiveness against the consequences of globally threatened COVID-19.
Methods
Ethical consideration and study approval
Ethical approval was taken from Bangladesh Medical Research Council after properly explaining the purpose and procedure. Similarly, the study approval was obtained from the Directorate General of Drug Administration and the Directorate General of Health Services of Bangladesh. Along with this effort, this study is also registered at NIH, US National Library of Medicine at ClinicalTrail.gov and has the registration no: NCT04402203. Having ethical and clinical approval, we took written consent from each of the respondents after sharing the study purpose.
Sample collection and diagnostic process
We collected nasopharyngeal swabs following the appropriate sample collection procedure for the COVID test using RT-PCR. Viral ribonucleic acids (RNAs) were extracted from the respiratory samples using the QIAamp Viral RNA Kit (Qiagen, Heiden, Germany) using a commercial kit specific for SARS-CoV-2 detection (A*Star Fortitude Kit 2 for COVID-19 Detection, Singapore). Similarly, the other related samples were collected and tested following appropriate methods.
Study setting and study population
This preliminary double-blinded randomized controlled trial (RCT) study was conducted among COVID-19 patients admitted to four hospitals in Dhaka city, Bangladesh from May 2020 to July 2020. The hospitals were Mohanagar General Hospital, Mugda Medical College Hospital, Kurmitola General Hospital, and Dhaka Medical College Hospital. These are the reputed government hospitals from where people have been receiving general health services for a long time. However, following the government guidance, these hospitals have also been ensuring COVID-19 services from the beginning of the epidemic in Bangladesh. The services include COVID-19 screening, diagnosis, confirmation, isolation, treatment, follow-up tests, and evaluation of treatment outcomes. In the beginning, a total of 126 COVID-19 patients were selected for the initial screening for this study; and then 66 were excluded due to RT-PCR negative results. Finally, 50 COVID-19 hospitalized patients were enrolled for RCT following the justification of clinical features and confirmation by RT-PCR. The enrolled patients were equally divided into two groups, such as the study group and the placebo group. The study group received FPV plus SoC and was identified as group A. Similarly, the placebo group received placebo plus SoC and was identified as group B. Along with socio-demographic and behavioral factors, the clinical features and diagnostic results were strictly evaluated with guidelines. Subsequently, post-treatment surveillance was also conducted.
Study procedure
A total of 50 patients were selected following trial criteria and patient consent. The selected patients' age range was between 18 and 65 years. For blinding, a statistician who was not involved in the trial did randomization by a computerized randomization table. Participants were randomly assigned (1:1) either to receive FVP or a placebo. The investigators and study participants were masked to subgroup assignments until the study was completed. It was noted that the composition of the drug and placebo was prepared by Beacon Pharmaceuticals Ltd, Dhaka, Bangladesh. In this study, Group-A received oral FPV following recommended dosage twice daily (Pharmaceuticals and Medical Devices Agency, 2011). Group B received a placebo which was indistinguishable from FPV following the same dosage, timing, and duration. The treatment-related other efforts, such as oxygen inhalation, oral or intravenous rehydration, electrolyte correction, antipyretics, analgesics, antibiotics, antiemetic drugs, and medication for any concomitant diseases were equally provided. Baseline clinical symptoms and laboratory findings were monitored and carefully recorded on day 0(baseline), and follow-up was continued on days 4, 7, and 10. The patient’s condition was also monitored by measuring hematological and biochemical parameters such as WBC, neutrophil, lymphocyte count, resting blood sugar, serum uric acid, and serum transaminases. The drug's adverse effects such as nausea, vomiting, diarrhea, jaundice, skin rash, anemia, vertigo, anosmia, and other effects were also carefully monitored following same-day intervals. The efficacy of the treatment was assessed by the time of viral clearance and the improvement rate of chest X-rays on days 4, 7, and 10 compared to the baseline condition (day 0). The term “Viral clearance” was defined as the presence of two consecutive negative results with PCR in 24 h.
Inclusion and exclusion criteria
Patients who had symptoms of COVID for at least 7 days, were aged between 18 and 65 years, and were not pregnant at the time of the study were included.
Patients who had moderate to severe clinical conditions such as a resting respiratory rate greater than 30 per minute, oxygen saturation below 93%, oxygenation index < 300 mmHg, respiratory failure, shock, organs failure, chronic liver and kidney diseases reaching end-stage, high serum uric acid level, along with patients at ICU, or patients with a previous history of allergic reactions to FPV, pregnant and lactating women, hypertensive patients and patients who were taking calcium channel blocker were all excluded from this study.
Outcome measure
Patient’s physical improvement with negative test results by RT-PCR and improvement by X-ray corresponding.
Statistical analysis
Statistical Package of Social Sciences (SPSS), IBM version 23 was used to analyze the relevant data. The value p < 0.05 was considered significant. The Kaplan-Meier curve presented the cumulative survival rate. The hazard ratio (HR) between two groups after adjusting for gender and age was calculated using Cox's proportional hazards model.
Results
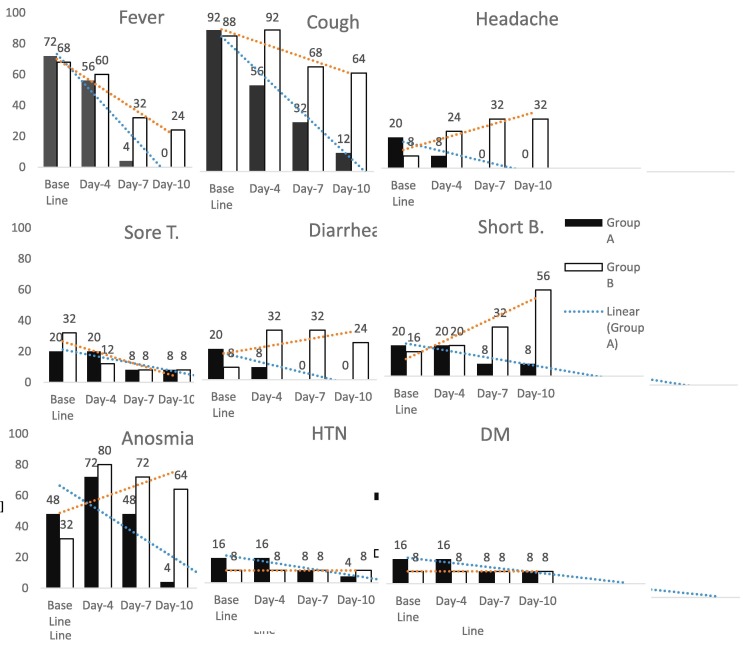
In this study, 66% were male, 32% were smokers and around one-third were service holders. The mean age of the total participants was 37.75 years. Most of the COVID-19 transmission (42%) occurred from family members [Table 1 ]. Among the group A patients, fever was present in 72%, caugh in 92%, headache, diarrhea and shortness of breath in 20% each, insomnia in 48%, hypertension and diabetes in 16% each were observed as baseline symptoms. Group B also had similar levels of fever, shortness of breath and caugh, but had lower incidence of headache, diarrhea, insomnia and higher incidence of sore throat at baseline. We found a comparatively higher reduction of symptoms among group A than group B on days 4, 7, and 10 respectively. But in case of headache, shortness of breath and insomnia, a linear increase was observed among group B participants in later days [Fig. 1 ]. The X-ray showed that among the FPV group, patients did not face worsening pneumonia. Among the pneumonia patients, a remarkable improvements of X-ray findings (47.4%) were noticed in Group A compared to Group B (no improvement at all) (p = 0.001) after four days of treatment (Table 2 ). After seven days of treatment, the chest X-ray of Group A showed 73.68% improvement compared to 25% improvement found in Group B (p = 0.009). Finally, 94.7% of patient’s lung condition was improved in FVP treated group which is significantly higher than that of the control group (50% improvement; p = 0.006). Half of the drug untreated patient’s (eight out of 16) lung condition of Group B remained constant or worsen [Table 2].
Table 1.
Demographic Characteristics of patients. (n = 50, equally divided into two groups).
| Group A | Group-B | Total (n = 50) | p-value | |
|---|---|---|---|---|
| Age in years (Mean ± SD) | 37.96 ± 11.45 | 37.54 ± 10.18 | 37.75 ± 10.73 | 0.893 |
| Gender | ||||
| Male | 16 (64.0%) | 17 (68.0%) | 33 (66.0%) | 0.765 |
| Female | 9 (36.0%) | 8 (32.0%) | 17 (34.0%) | |
| Smoking status | ||||
| Yes | 9 (36.0%) | 7 (28.0%) | 16 (32.0%) | 0.540 |
| No | 16 (64.0%) | 18 (72.0%) | 34 (68.0%) | |
| Family contact history | ||||
| Yes | 11 (44%) | 10 (40%) | 21 (42%) | 0.876 |
| No | 14 (56.0%) | 15 (60.0%) | 29 (58%) | |
| Occupation | ||||
| Service Holder | 10 (40%) | 9 (36%) | 19 (38%) | |
| Others | 15 (60%) | 16 (64%) | 31 (62%) |
Fig. 1.
Baseline characteristics of the patients with comparison after the intervention. (n = 50).
Table 4.
Effect of FVP on hematological and biochemical parameters.
| Baseline value |
Day-4 |
Day-7 |
Day-10 |
|||||
|---|---|---|---|---|---|---|---|---|
| Group-A Mean (95% CI) | Group-B Mean (95% CI) | Group-A Mean (95% CI) | Group-B Mean (95% CI) | Group-A Mean (95% CI) | Group-B Mean (95% CI) | Group-A Mean (95% CI) | Group-B Mean (95% CI) | |
| WBC | 6.5 (5.7–7.3) |
6.4 (5.3–7.6) |
7.8 (7.2–8.5) |
7.4 (6.3–8.4) |
8.2 (7.3–9.1) |
8.0 (6.9–9.1) |
7.8 (6.8–8.8) |
8.0 (7.1–9.0) |
| Neutrophil | 62.8 (57.2–68.3) |
56.2 (49.1–63.2) |
65.8 (61.4–70.1) |
62.3 (58.5–66.1) |
60.6 (53.7–67.7) |
63.2 (59.9–66.5) |
63.4 (59.4–67.4) |
64.1 (61.3–66.9) |
| Lymphocyte | 31.8 (26.7–36.9) |
35.1 (30.3–39.9) |
30.0 (25.6–34.5) |
31.7 (27.1–36.2) |
31.8 (28.8–34.8) |
31.8 (28.8–34.8) |
30.9 (27.1–34.7) |
30.7 (27.9–33.5) |
| ALT | 39.1 (29.2–49.0) | 39.3 (24.7–53.9) |
46.2 (32.4–59.9) |
50.8 (37.2–64.4) |
42.4 (30.9–53.8) | 53.9 (36.5–71.3) | 39.4 (27.6–51.8) | 50.5 (27.9–33.5) |
| AST | 35.9 (26.7–45.1) | 32.8 (24.8–40.8) | 33.2 (24.9–41.7) | 35.8 (28.6–43.1) | 32.2 (25.9–38.4) | 33.6 (26.6–40.7) | 28.6 (23.4–33.7) | 33.3 (26.8–39.7) |
| Serum UA | 5.5 (4.8–6.1) |
5.8 (5.3–6.4) |
8.1 (7.1–8.9) |
6.5 (5.8–7.2) |
7.8 (6.8–8.3) |
7.2 (6.1–8.9) |
8.0 (6.9–9.2) |
6.9 (6.0–7.8) |
| CRP | 13.5 (11.2–15.7) |
10.7 (8.9–12.5) |
13.7 (11.7–15.6) |
11.4 (9.9–13.1) |
12.9 (11.5–14.3) |
13.4 (11.1–15.7) |
12.9 (10.8–15.0) |
12.3 (10.4–14.2) |
| RBS | 6.5 (5.1–7.9) |
5.6 (4.8–6.3) |
6.3 (5.3–7.2) |
5.9 (5.4–6.5) |
6.0 (5.0–7.0) |
6.2 (4.5–8.0) |
6.0 (4.9–7.2) |
6.1 (4.8–7.3) |
Table 2.
Chest X-ray Findings of Baseline Pneumonia patients. (n = 50 equally divided into two groups).
| Baseline |
Day 4 |
Day 7 |
Day 10 |
|||||
|---|---|---|---|---|---|---|---|---|
| Group-A, n = 25, % | Group-B, n = 25, % | Group-A, n = 19, % | Group-B, n = 16, % | Group-A, n = 19, % | Group-B, n = 16, % | Group-A, n = 19, % | Group-B, n = 16, % | |
| Improved | 19 (76.0) | 16 (64.0) | 9(47.36) | 0 (0.0) | 14 (73.68) | 4 (25.0) | 18 (94.73) | 8 (50.0) |
| Worsen | – | – | 2 (10.5) | 3 (16) | 0 (0%) | 1 (6.25%) | 0 (0.0) | 1 (6.25) |
| p-value | 0.001 | 0.009 | 0.006 | |||||
Regarding virus clearance, at day four, 48% patients of FVP group (12 out of 25 patients) became cle.red from SARS-CoV-2. In contrast, there was no clearance in the control group. After 7 day of treatment, the cumulative viral clearance was found to be 76% in FVP group compared to 37.5% in placebo treated control group (p = 0.005). Total no of patients became freed from SARS-CoV-2 was 24 out of 25 (96%) which was significantly higher than that of the control group (13 out of 25; p = 0.001). Total 12 (48%) patients of control group still remained infected after 10 days of study [Table 3 ].
Table 3.
Cumulative Viral Clearance at different time point.
| Day 4 | Day 7 | Day 10 | No clearance | |
|---|---|---|---|---|
| Group A (n = 25) | 12 (48.0%) | 19 (76%) | 24 (96%) | 1 (4.0%) |
| Group B (n = 25) | 0 (0%) | 9 (36%) | 13 (52%) | 12 (48%) |
| P value | 0.001 | 0.005 | 0.001 | 0.002 |
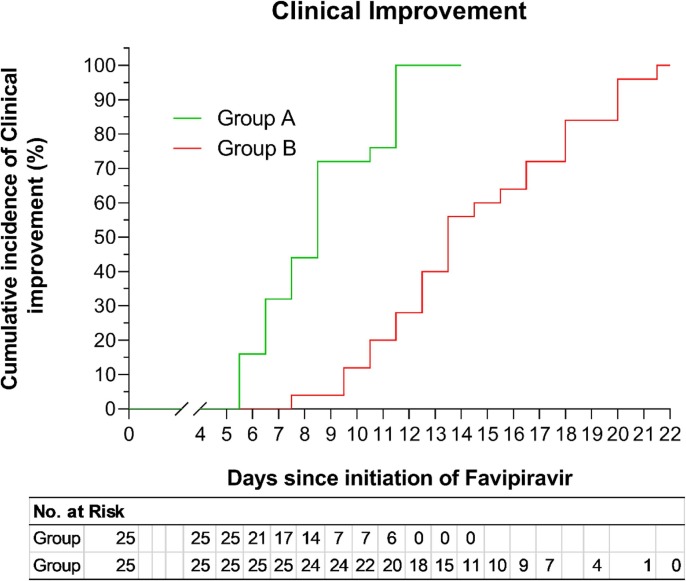
Hematological and Biochemical parameters such as total WBC count, neutrophil and lymphocyte counts, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), uric acid, C-reactive protein (CRP) and resting blood sugar (RBS) were examined in different time intervals of both group and the results were compared with the baseline values and within groups. There were no significant differences in the hematological parameters such as WBC count, neutrophil and lymphocyte counts in Group A and Group B patients in different time points. Liver transaminases levels were also stable in FVP treated group (average ALT ranges 39.4–46.2; AST 28.2–32.8). Although a slight increase of ALT was observed in the control group (mean 39.4 vs 50.5). There was a slight increase of uric acid level in FVP group compared to control (mean 8.0 mg/dl vs 6.9 mg/dl). Slightly elevated CRP level did not change in the study period and the resting blood sugar (RBS) also remained the same [Table 4 ]. Culmulative incidence of clinical improvement was ascertained and recorded by hospital discharge history by Kaplan-Meier survival curve, and it was observed that by days 11–12, all patients of group A had clinical improvement [Fig. 2 ]. These findings indicate the efficacy and effectiveness of FPV on viral clearance. Adverse effects in drug therapy were noted at several time points (0, 4, 7, and 10 days) and compared with control group. Nausea was present in 28% in group A and 20% in group B at the baseline, but it was not clear whether the adverse effects were because of drugs or infection. Nausea improved slightly faster among group B patients. Diarrhoea was noted for two patients which was also present before starting the drug and in control group too. These adverse effects disappeared gradually. Bleeding, jaundice, skin rash, liver damage, anemia, vertigo and other notable side effects were not found for FVP treatment. Although two patients of both groups presented with vertigo at baseline, group A had no vertigo patients by day 7 [Table 5 ].
Fig. 2.
Comparison of survivor function in two treatment groups by Kaplan-Meier survival curve. Data has been presented as the cumulative incidence of clinical improvement ascertained by hospital discharge of patients. Group A (n = 25), Group B (n = 25).
Table 5.
Assessment of Adverse effects of patients of both groups.
| Baseline value |
Day-4 |
Day-7 |
Day-10 |
|||||
|---|---|---|---|---|---|---|---|---|
| Grp-AB | Grp-BC | Grp-A | Grp-B | Grp-A | Grp-B | Grp-A | Grp-B | |
| Nausea | 7 (28%) | 5 (20%) | 11 (44%) | 9 (36%) | 4 (16%) | 4 (16%) | 3 (12%) | 1 (4%) |
| Vomiting | 2 (8%) | 2 (8%) | 4 (16%) | 2 (8%) | 2 (8%) | 1 (4%) | 1 (4%) | 0 (0%) |
| Diarrhea | 3 (12%) | 2 (8%) | 0 (0%) | 1 (4%) | 2 (8%) | 2 (8%) | 1 (4%) | 0 (0%) |
| Bleeding | 1 (4%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Jaundice | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Skin rash | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Liver damage | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2(8%) | 0 (0%) | 1 (4%) |
| Anemia | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Vertigo | 2 (8%) | 2 (8%) | 2 (8%) | 2 (8%) | 0 (0%) | 2 (8%) | 0 (0%) | 1 (4%) |
| Anosmia | 1 (4%) | 0 (0%) | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Baseline characteristics of patients and conditions after intervention. (n = 50 equally divided into two groups).
Discussion
This randomized, double-blinded preliminary placebo-controlled clinical trial was conducted in mild to moderate COVID-19 patients. We used antiviral prodrug FVP on COVID-19 patients. This drug has also been used against influenza and other RNA virus-related diseases (Nagata et al., 2015, Delang et al., 2018, Sleeman et al., 2010). In this study, the demographic features and baseline characteristics of both the study group and the placebo group were almost similar. However, we found a higher viral clearance among the FPV group than the placebo group starting from day four, and this high clearance rate continued at follow-ups on days seven and ten. On day ten, around 96% of viral clearance was observed among the FPV group, whereas only 52% clearance was observed among the placebo group. This finding indicates the drug's efficacy and effectiveness against COVID-19. We observed that after four-day treatment, about half of the patients were recovered from infection with 48% viral clearance among the FPV group while there was no viral clearance (0%) and no recovery progress among the placebo group, and this difference was statistically significant (p < 0.001). The above results were also supported by the findings of the chest X-ray on COVID-19 pneumonia and non-pneumonia patients. Our findings were validated by the findings of other studies (NIH US, 2020, Roser et al., 2020, COVID-19, 2020). Currently, there are at least 30 clinical trials of FVP registered on ClinicalTrials.gov of NIH (NIH US, 2020). Most of them are in progress and only a few of them have been completed. So far, the results of one open-label controlled clinical trial conducted in China using combined therapy of FVP and interferon-alpha (INF-a) compared to Lopinavir/Ritonavir (LPV/RTV) plus INF-a in the control group has been published (Cai et al., 2020). In this trial, FVP treated patients showed early recovery and significant improvement in chest X-rays. Several clinical trials were completed in China, India, and Russia which also showed similar results (COVID-19, 2020, Chen et al., 2020,, Trial site news, 2020). Most of them conducted an open-label controlled trial, which was different from our preliminary double-blinded placebo-controlled randomized clinical trial. A meta-analysis by Hassanipour showed significant clinical improvement and higher viral clearance similar to the findings of our study (Hassanipour et al., 2021).
The observed effect of FVP in COVID-19 patients might be a reduction of viral load through inhibition of viral replication (Furuta et al., 2013). The prodrug FVP becomes activated via ribosylation and phosphorylation to its active form favipiravir ribofuranosyl-5′-triphosphate (T-705-RTP), which inhibits RNA dependent RNA polymerase (RdRp) (Furuta et al., 2017). FVP was a potential RdRp inhibitor that decreased the viral load earlier than the control group, which resulted in the clinical improvement in X-ray findings of the patients. FVP exhibited no notable adverse effects, and none of the patients needed to discontinue the drug. Although transient nausea and vomiting were observed in a few patients, it was unclear whether these adverse effects were a result of the medication or the infection. The other studies also found a similar safety profile of FPV (Pilkington et al., 2020). However, we observed a slight increase in uric acid levels, which was consistent with other studies (COVID-19, 2020). This increase in uric acid level might be due to the decreased tubular secretion of uric acid mediated by the inhibitory action of FVP and its metabolite on organic acid transporters OAT1 and OAT3 (Pharmaceuticals and Medical Devices Agency, 2011). However, similar to other studies, the FVP induced hyperuricemia was transient and did not produce any clinical manifestation (Pharmaceuticals and Medical Devices Agency, 2011, Du and Chen, 2020).
Limitations of the study
This study was conducted with a small size, as we excluded a significant number of patients who did not meet eligibility criteria and also there was not much patient in Bnagladesh when the trial was conducted. The patients' age was lower than usual, and before hospitalization, the patients' frequent symptoms and physical conditions were not properly analyzed.
Conclusion
In this trial, FPV was found to accelerate the viral clearance and clinical improvements in chest X-rays markedly. Subsequently, FPV displayed no considerable adverse effects. However, we feel that a large randomized controlled trial should be conducted to make any conclusive decision.
Author Contributions
SMAR and AK designed and wrote the study protocol. AK was the Principal Investigator of this trial. SMAR played advisory roles. AK, NAA, KAKA, MBA, and MTM supervised the clinical trial in different hospitals. MBA. MTM, GMM, MRA, MA, MAK, and SRD were involved in clinical intervention and data collection. SMAR wrote the manuscript. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We acknowledge the effort of Beacon Pharmaceutical Limited, Bangladesh for developing FPV and placebo. We are grateful to the hospital authorities for their support and cooperation. We are showing the highest honor to patients who provided consent and participated in this preliminary trial study. Finally, we acknowledge BMRC, DGDA, DGHS, and registering authorities who had approved the study at the local and international level.
Funding
Beacon Pharmaceuticals Limited, Dhaka, Bangladesh.
References
- Alam S., Kamal T.B., Sarkar M.M.R., Zhou J.-R., Rahman S.M.A., Mohamed I.N. Therapeutic effectiveness and safety of repurposing drugs for the treatment of COVID-19: position standing in 2021. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.659577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., et al. Experimental treatment with favipiravir for COVID-19: An open-label control study. Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.03.007. (online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callender L.A., Curran M., Bates S.M., Mairesse M., Weigandt J., Betts C.J. The impact of Pre-existing comorbidities and Therapeutic Interventions on COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01991. Accessed September 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., et al. Favipiravir versus arbidol for COVID-19: A randomized clinical trial. medRxiv. 2020 doi: 10.1101/2020.03.17.20037432. Posted on medRxiv March 20. 2020. [DOI] [Google Scholar]
- COVID-19: Glenmark’s favipiravir shows encouraging results in phase 3 clinical trial. The Indian Express. https://www.newindianexpress.com/nation/2020/jul/23/covid-19-glenmarks-favipiravir-shows-encouraging-results-in-phase-3-clinical-trial-2173500.html. Accessed Septembe 23r, 2020.
- Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delang L., Abdelnabi R., Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral. Res. 2018;153:85–94. doi: 10.1016/j.antiviral.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Du Y.-X., Chen X.-P. Favipiravir: Pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin. Pharmacol. Ther. 2020;108(2):242–247. doi: 10.1002/cpt.1844. [DOI] [PubMed] [Google Scholar]
- Furuta Y., et al. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100(2):446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanipour S., Arab-Zozani M., Amani B., Heidarzad F., Fathalipour M., Martinez-de-Hoyo R. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-90551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.J., Rahman S.M.A. Repurposing therapeutic agents againsts SARS-CoV-2 infection: most promising and neoteric progress. Expert Rev. Antiinfective Therapy. 2021;19(8):1009–1027. doi: 10.1080/14787210.2021.1864327. [DOI] [PubMed] [Google Scholar]
- Hossain M.J., Hridoy A., Rahman S.M.A., Ahmad F. Major depressive and Generalized anxiety disorders among university students during the second wave of COVID-19 outbreak in Bnagladesh. Asia Pac. J. Pubic Health. 2021;33(5):676–678. doi: 10.1177/10105395211014345. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y.i., Zhang L.i., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L.i., Xie J., Wang G., Jiang R., Gao Z., Jin Q.i., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal Filho W., Brandli L.L., Lange Salvia A., Rayman-Bacchus L., Platje J. Covid-19 and the UN Sustainable Development Goals: Threat to Solidarity or an Opportunity? Sustainability. 2020;12(13):5343. [Google Scholar]
- Li G., Clercq E.D. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- Lu H., Stratton C.W., Tang Y.-W. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J. Med. Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T., Lefor A.K., Hasegawa M., Ishii M. Favipiravir: a new medication for the ebola virus disease pandemic. Disaster Med. Public Health Preparedness. 2015;9(1):79–81. doi: 10.1017/dmp.2014.151. [DOI] [PubMed] [Google Scholar]
- Nicola M., et al. The Socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH US. National Library of Medicine ClinicalTrial.gov. https://clinicaltrials.gov/ct2/home. Accessed September 5, 2020.
- Pharmaceuticals and Medical Devices Agency, Report on the deliberation results – avigan. Japan; Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau, 2011. Available at: http://www.pmda.go.jp/files/000210319.pdf. Accessed September 20, 2020.
- Pilkington V., Pepperrell T., Hill A. A review of the safety of favipiravir-a potential treatment in the COVID-19 pandemic? J. Virus Erad. 2020;6(2):45–51. doi: 10.1016/S2055-6640(20)30016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roser M, et.al. Coronavirus Disease (COVID-19)-Statistics and Research. Published online at OurworldData.org. retrieved from https://ourworldindata.org/coronavirus. Accessed 8 Sep 2020.
- Sleeman K., Mishin V.P., Deyde V.M., Furuta Y., Klimov A.I., Gubareva L.V. In vitro antiviral activity of favipiravir (T-705) against drug-resistant influenza and 2009 a (H1N1) viruses. Antimicrob. Agents Chemother. 2010;54(6):2517–2524. doi: 10.1128/AAC.01739-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trial site news. Russia ministry of health approves avifavir (favipiravir) for COVID-19 patients—cuts duration of illness by over 50 %. https://www.trialsitenews.com/russia-ministry-of-health-approves-avifavir-favipiravir-for-covid-19-patients-cuts-duration-of-illness-by-over-50/. Accessed September 1, 2020).
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W.u., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B.o., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P., Murdoch D.R. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395(10229):1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worldometer: https://www.worldometers.info/coronavirus/. Accessed 5th April, 2021.
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y.i., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China:a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]