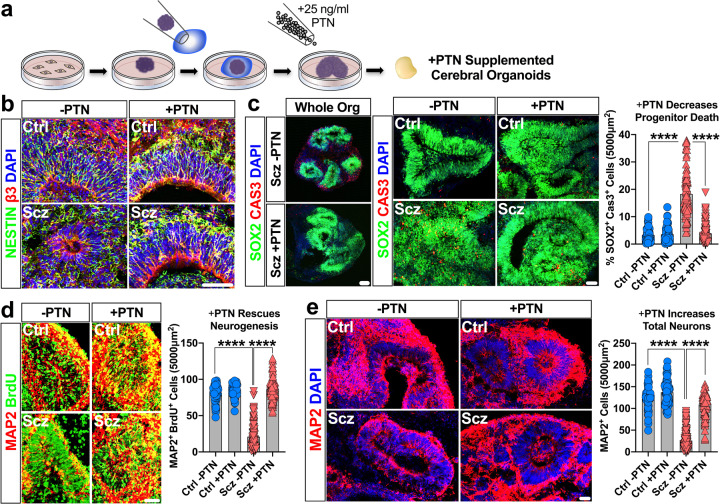
Fig. 5. PTN rescues both survival and neurogenesis in Scz Organoids.
a Schematic of PTN-supplementation regime for rescue experiments. Proteomics revealed a decrease in total PTN expression in Scz organoids (Fig. 2e). Similarly, scRNA-Seq revealed that Scz organoids exhibited lower PTN gene-expression in progenitors and neurons (Fig. 3f, g). We identified that organoids supplemented with 25 ng/ml PTN (a concentration for growth factor supplements) resulted in viable tissue without gross morphological evidence of deterioration. This subsequently permitted a laboratory-controlled examination of PTN’s role in intrinsically arising neuropathology of Scz in self-developing human-derived neural tissue. b–c, PTN treatment promotes progenitor survival in Scz organoids. PTN is expressed in most cell type clusters in organoids (Fig. 3f) but is particularly enriched in neural progenitors (Fig. 3g). Post treatment, the ventricular zones of PTN-supplemented Scz organoids often appeared enriched for Nestin (b). PTN treatment rescued progenitor death in the ventricular zones of Scz organoids to levels consistent with Ctrl cultures (panel c; Vehicle Ctrl n = 69 fields, n = 28 organoids, and n = 5 independent Ctrl lines; PTN-supplemented Ctrl n = 70 fields, n = 30 organoids, and n = 5 independent Ctrl lines; Vehicle Scz n = 90 fields, n = 44 organoids, and n = 9 independent Scz lines; PTN-supplemented Scz n = 85 fields, n = 41 organoids, and n = 9 independent Scz lines). Enlarged, whole-organoid, images are also provided in the Supplementary Material (see Fig. S9). PTN treatment also broadly increased new-born cell survival in Scz organoids (see Fig. S10). These data suggest that PTN exerts neurotrophic-like effects on neural progenitors in organoids, and that low PTN expression in Scz organoids regulates neural progenitor cell death. As with our prior mechanistic rescue figure, raw data are graphed here for phenotype transparency and each data point on graphs reflects an independent ventricular zone (see Fig. S11 for averaged data). d–e, PTN supplementation restores neuronal differentiation in Scz organoids. We next sought to determine if PTN levels contribute to neurogenesis phenotype in Scz organoids. To do this, we time-locked our 24 h-7d BrdU pulse-chase paradigm to coincide with commencement of PTN treatment. As in previous experiments, Scz organoids exhibited disrupted neuronal differentiation relative to Ctrl organoids. However, PTN treatment rescued the disrupted neurogenesis of Scz organoids. PTN supplementation restored the number of MAP2+ BrdU+ neurons in Scz organoids to levels consistent with Ctrl cultures (panel d; Vehicle Ctrl n = 75 fields, n = 26 organoids, and n = 5 independent Ctrl lines; PTN-supplemented Ctrl n = 79 fields, n = 27 organoids, and n = 5 independent Ctrl lines; Vehicle Scz n = 130 fields, n = 45 organoids, and n = 10 independent Scz lines; PTN-supplemented Scz n = 106 fields, n = 42 organoids, and n = 10 independent Scz lines). Thus, in addition to promoting progenitor survival, PTN-supplementation also promotes neuronal differentiation in Scz organoids. Lastly, and consistent with all prior phenotypes, PTN treatment increased total MAP2+ neuron numbers in Scz cortical fields as expected (panel e; Vehicle Ctrl n = 58 fields, n = 19 organoids, and n = 4 independent Ctrl lines; PTN-supplemented Ctrl n = 67 fields, n = 25 organoids, and n = 4 independent Ctrl lines; Vehicle Scz n = 150 fields, n = 57 organoids, and n = 11 independent Scz lines; PTN-supplemented Scz n = 97 fields, n = 41 organoids, and n = 10 independent Scz lines). Together, these experiments established that PTN mechanistically contributes to neuron numbers in 3D cortical assemblies within Scz organoids. Each data point on graphs reflects an independent, non-overlapping, cortical field, for phenotype transparency, with averaged data provided in Supplementary Material (see Fig. S11). ****p < 0.0001. Error bars reflect Standard Error of the Mean. Scale bar: 60 µm. Ctrl control, Scz schizophrenia.