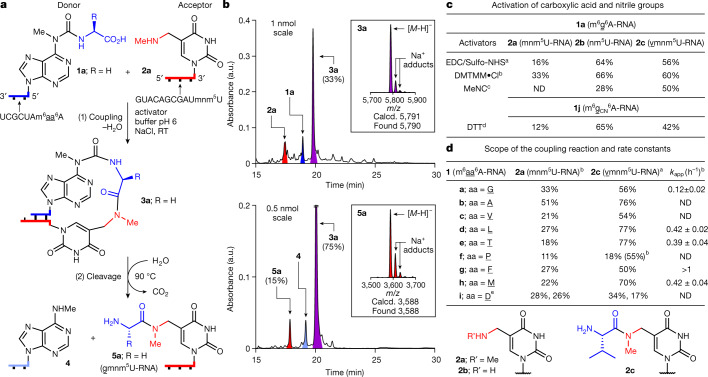
Fig. 2. Peptide synthesis on RNA with terminal (m)nm5U and m6aa6A nucleotides.
a, Reaction scheme for 1a (5′-m6g6A-RNA-3′) and 2a (3′-mnm5U-RNA-5′) with coupling (1) and cleavage (2). b, HPLC chromatograms of the crude reaction mixtures, obtained after coupling of 1a with 2a using DMTMM·Cl (see reaction condition b) and cleavage of 3a (100 mM MES buffer pH 6, 100 mM NaCl, 90 °C, 6 h). HPLC peaks of RNAs are coloured: donor in blue; acceptor in red; hairpin-type intermediate in purple; and cleaved donor strand in pale blue. The insets show MALDI-TOF data (negative mode) of the isolated products 3a and 5a. Calcd., calculated. c, Coupling results obtained with different activators for 1a and 1j with 2a–2c. d, Coupling reactions with different donors 1a–1i and acceptors 2a,2c, and apparent rate constants (kapp) of selected coupling reactions with 2c. All coupling reactions were carried out using a concentration of 50 μM for 1a–1j and 50 μM for 2a–2c (100 mM NaCl, 25 °C). a50 mM EDC/Sulfo-NHS (100 mM MES buffer pH 6, 24 h). b50 mM DMTMM·Cl (100 mM MES buffer pH 6, 24 h). c50 mM MeNC (50 mM DCI buffer pH 6, 5 days). d50 mM DTT (100 mM borate buffer pH 8, 24 h). eThe two yields with 1i (aa, D) describe the reaction of the aspartic acid α-COOH and of the side chain COOH. An assignment was not performed. RT, room temperature; ND, not determined.