Fig. 3.
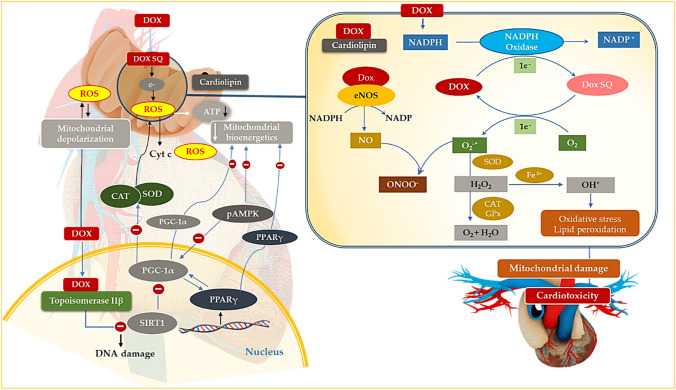
Doxorubicin-induced mitochondrial dysfunction. Concisely, NADH dehydrogenase (situated in complex I of the electron transport chain (ETC)) catalyzes the Dox redox quinone cycle to form a semiquinone. The semiquinone readily undergoes auto-oxidation by donating an electron to molecular oxygen (O2) to generate ROS (O•– 2 and H2O2) and lipid peroxides. Increased ROS, accompanied with a paucity of antioxidants, potentiates mitochondrial-induced oxidative damage. Dox also binds to topoisomerase IIβ (Top IIβ) to induce DNA damage and the subsequent downregulation of bioenergetic genes (adenosine monophosphate-activated protein kinase (AMPK), peroxisome proliferator-activated receptor-γ (PPARγ) coactivator-1α and β (PGC-1α and β)). Impaired bioenergetics with concomitant oxidative stress disrupt the mitochondrial gradient to induce mitochondrial dysfunction