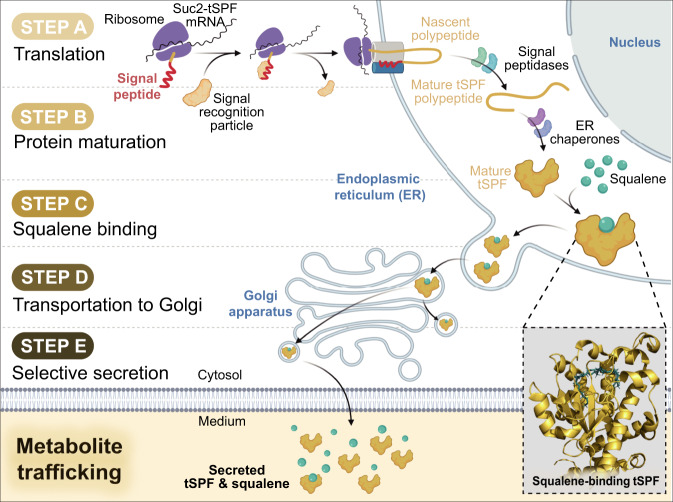
Fig. 1. Metabolite trafficking pathway for selective terpene secretion.
By combining a squalene-binding protein, a lipid-binding domain of supernatant protein factor (tSPF), and the export signal peptide of sucrose transport protein (Suc2), we systematically designed a fusion protein, Suc2-tSPF. Lipophilic squalene could be loaded into the Suc2-tSPF and transported into the extracellular milieu, across the otherwise impermeable membrane. To achieve selective terpene secretion, Suc2-tSPF performs a series of coordinated actions. During Suc2-tSPF mRNA translation, the N-terminal signal peptide is bound to a signal recognition particle for co-translational translocation, and the complex of the ribosome with the partially translated Suc2-tSPF is transferred to the endoplasmic reticulum (ER) membrane (step a). A nascent polypeptide is synthesized, and the signal peptide is cleaved by signal peptidases in the ER lumen, leaving behind a tSPF polypeptide (step b). The tSPF polypeptide is further modified by ER chaperones to form a mature tSPF protein, which captures squalene, the target metabolite (step c). Once squalene succeeds in hitchhiking, the tSPF protein, encapsulated by a transport vesicle, is subsequently transported to a Golgi apparatus (step d). To finalize squalene trafficking, a secretory vesicle exports the squalene-carrying tSPF into the extracellular space (step e).