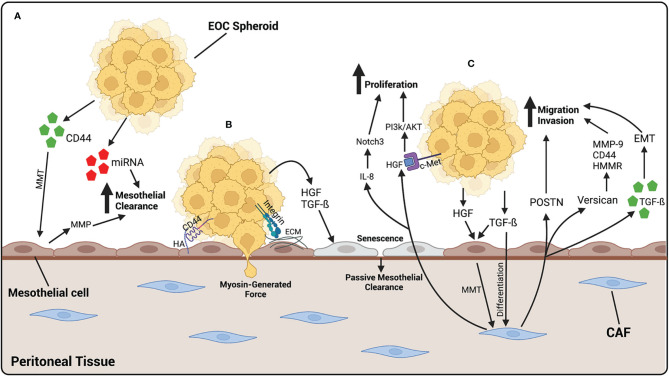
Figure 3.
EOC metastasizes to the peritoneal tissue, where it binds and invades through mesothelial cells. (A) EOC cells release CD44 which cause MMT of mesothelial cells, giving them a more fibroblast-like function. MMP, released by mesothelial cells, and miRNA-containing exosomes, released by EOC cells, increase mesothelial clearance of cancer cells. (B) EOC spheroids adhere to the mesothelium by the binding of CD44 on EOC cells to HA on mesothelial cells or integrins binding to ECM components. Once adhered, EOC cells undergo mesothelial clearance, either by using myosin-generated force, or in a more passive way by generating cellular senescence. HGF and TGF-β released by EOC cells initiates senescence of mesothelial cells, leading to an increase in passive mesothelial clearance. (C) Fibroblasts differentiate into CAFs by TGF-β released by EOC cells. HGF and TGF-β released by EOC also contributes to the activation of CAFs, by initiating MMT in mesothelial cells. Once activated, CAFs release POSTN and versican which contribute to the migratory and invasive abilities of EOC cells. CAFs also release exosomes containing TGF-β which increase the ability of cancer cells to undergo EMT, contributing to an increased ability to invade. CAFs also release HGF which binds to c-Met on EOC cells, activating the PI3k/AKT pathway leading to an increase in proliferation. IL-8 released by CAFs activates the Notch3 pathway also leading to an increase in EOC cell proliferation. Created with BioRender.com.