Abstract
Background
The frozen elephant trunk technique is useful in aortic arch repair; however, some adverse events are associated with the Frozenix J-graft. We designed a technique to prevent these adverse events and achieve easy anastomosis (Total Exclusion of the Non-Stent part of Frozenix using an Everting anastomosis [TENSE]), and we assessed the outcomes of this technique in the present study.
Methods
From April 2017 to May 2021, 44 patients with aortic arch disease underwent TENSE, in which the proximal stump of the stent part of Frozenix was matched to the distal anastomosis end between the left common carotid and left subclavian arteries.
Results
The median age of the patients (35 men, 9 women) was 76.5 years. The predicted mortality and morbidity rates were 10.0% and 40.2%, respectively, according to the JapanSCORE II. Two patients (4.5%) died of aneurysm rupture and interstitial pneumonia, respectively, during hospitalization. Four patients (9.1%) who developed postoperative cerebral infarction had a previous cerebral infarction (P=0.010). No patients developed spinal cord complications or Frozenix kinking. Follow-up computed tomography showed no endoleaks or aneurysmal dilatation, although one patient had possible distal stent graft-induced new entry.
Conclusions
Our strategy provided good early outcomes without spinal cord complications or Frozenix kinking in patients with aortic arch disease. Continuous follow-up is needed to avoid missing distal changes.
Keywords: Dissecting aortic aneurysm, aortic arch aneurysm, frozen elephant trunk technique, J-graft open stent graft, total arch replacement
Introduction
The frozen elephant trunk (FET) technique is commonly used for completion in the treatment of extensive aortic arch aneurysms (1-3). This technique is also used in patients with acute type A aortic dissection to close the entry of the proximal descending aorta and stabilize the intima by decreasing pressure in the false lumen (4,5). The FET technique for both of these conditions of the aortic arch has provided good early and long-term results with lower morbidity than conventional aortic arch replacement (2,3). In Japan, use of the Frozenix® J-graft (Japan Lifeline Inc., Tokyo, Japan) as part of the FET technique to treat aortic arch aneurysms and aortic dissection has been increasing since 2014 because neither the E-vita OPEN PLUS hybrid prosthesis (JOTEC GmbH, Hechingen, Germany) nor the Thoraflex hybrid prosthesis (Vascutek, Terumo, Inchinnan, Scotland) has received insurance approval in Japan (1).
Notably, the FET technique is associated with some complications, such as coarctation or kinking of the open stent graft; these complications are in turn associated with devasting adverse events that require additional intervention (1-4,6-8). In patients with an acute angulated or narrow aortic arch, kinking of the Frozenix J-graft may occur between the non-stent and stent parts after total arch replacement (TAR) with the FET technique (1,6). Based on the results of their multicenter study, Uchida et al. (1) recommended shortening the non-stent part to the greatest extent possible to prevent kinking of the Frozenix J-graft. Moreover, after deploying the Frozenix J-graft, the non-stent part is commonly distorted into folds and wrinkles, which makes anastomosing the vascular graft difficult. According to these findings, we considered total exclusion of the non-stent part to prevent kinking of the Frozenix J-graft and obtain good expansion of the proximal portion of the graft. Additionally, arch replacement can injure the recurrent laryngeal nerve, especially when manipulating the aortic arch distal to the left subclavian artery (1,2,4). Therefore, we designed a technique to facilitate easy anastomosis and prevent complications associated with the Frozenix J-graft with an arch translocation anastomosis technique. This technique is referred to as Total Exclusion of the Non-Stent part of the Frozenix J-graft with an Everting anastomosis (TENSE), and it can be used in patients with aortic arch disease.
The number of reports showing the outcomes of the Frozenix J-graft is still relatively small, and all such studies have been multicenter in nature. Furthermore, although shortening of the non-stent part and use of the arch translocation anastomosis technique may be well known, few reports have included both techniques. In this study, we assessed the results of TAR using the Frozenix J-graft with the TENSE technique for aortic arch disease at a single center. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1751/rc).
Methods
Study population
We retrospectively analyzed the data of 44 patients who underwent TENSE for aortic arch disease, including an extensive aortic arch, distal aortic arch aneurysm, and chronic dissecting aortic aneurysm, from April 2017 to March 2021 (4 patients in 2017, 10 in 2018, 15 in 2019, 14 in 2020, and 1 in 2021). Patients with acute aortic dissection were excluded from this study because compared with aneurysms, acute aortic dissection has a different pathology and requires different surgical strategies. We planned to perform secondary interventions in patients who required deployment of the Frozenix J-graft distal to T8 for aortic aneurysms that extended from the aortic arch to the distal descending aorta. We assessed the early outcomes of the TENSE technique.
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Ethics Board of Osaka City Medical School Hospital (Approval No. 4203), and written informed consent was obtained from all the patients. Preoperative comorbidities and perioperative complications were defined according to the Japan Cardiovascular Surgery Database (9).
Surgical technique
Conventional approach
Full medial sternotomy was performed under general anesthesia. After systemic heparinization, we established cardiopulmonary bypass with an arterial cannula to the ascending aorta as well as a two-stage cannula through the right atrial appendage (most commonly) or a bicaval venous cannula to the superior and inferior vena cava (in patients who required intracardiac operations). The body temperature was then cooled to <27 ℃ and measured at the pharynx. After performing aortic cross-clamping or moderate hypothermic circulatory arrest, antegrade and retrograde identical cold-blood cardioplegia were administered, followed by intermittent selective administration into the orifices of the coronary arteries or retrograde administration.
Minimal incisional approach
We performed TAR with the FET technique in patients with aortic arch disease via upper partial sternotomy under general anesthesia. We applied this technique in patients who underwent aortic arch aneurysmal repair alone if anatomically acceptable. We established cardiopulmonary bypass with an arterial cannula to the ascending aorta and a venous cannula to the right atrium through the right femoral vein. Vacuum-assisted venous drainage was applied, and the cardioplegia and operative procedures were identical to those in the conventional approach.
TENSE technique
We designed an arch translocation anastomosis technique for TAR involving the distal anastomosis site between the left common carotid and left subclavian arteries under moderate hypothermic circulatory arrest with conventional antegrade cerebral perfusion to reduce injury to the left recurrent laryngeal nerve and easily achieve hemostasis of the distal anastomosis.
When kinking of the Frozenix J-graft occurred after TAR with the FET technique for acute type A aortic dissection, the kink was often located between the non-stent and stent parts of the Frozenix J-graft (Figure S1) (6). This adverse event may result from a severely angulated arch preserved by arch translocation. Figure 1 shows TESE technique. TENSE involves two steps. First, the proximal stump of the stent part of the open stent graft is matched to the distal anastomosis end of the aortic arch by several sutures, with reinforcement using a Teflon felt strip (Figure 1B). Second, the branched vascular graft with an everted distal end is interpolated and anastomosed to the stump of the aortic arch fixed to the Frozenix J-graft [“turn-up” technique (10)] (Figure 1C). We chose the size and length of the Frozenix J-graft according to the preoperative computed tomography angiography (CTA) findings. In terms of surgical repair of dissecting aneurysms, the size of the Frozenix J-graft was 5% to 10% larger than the inner diameter of the true lumen, which was calculated by dividing the circumference of the true lumen by pi. However, 10% to 20% oversizing of the landing aorta is necessary in aortic arch aneurysm repair. The length of the stent part of the Frozenix J-graft was chosen according to the distance from the distal anastomosis site to the entry site with deployment in the relatively straight zone of the descending aorta or with a length of >3 cm of the distal landing in the descending aorta over the arch aneurysm to prevent endoleaks. The Frozenix J-graft was deployed proximal to the aortic valve level under transesophageal echocardiographic guidance. The orifice of the subclavian artery was closed by suturing and with a Weck® Hem-o-lok® Polymer Locking Ligation System (Teleflex Inc., Morrisville, NC, USA). Following distal anastomosis of the aortic arch, the left subclavian artery, left common carotid artery, and brachiocephalic artery were sequentially reconstructed. The left subclavian artery was reconstructed by direct or extra-anatomical bypass if this artery was located in a deep dorsal position.
Figure 1.
Schema of TENSE technique using the Frozenix J-graft. (A) Schema of distal aortic arch aneurysm. (B) Deployment of the Frozenix J-graft with matching the proximal stump of the stent part to the distal anastomosis end of the aortic arch. (C) Anastomosis of the branched vascular graft with an everted distal end, the Frozenix J-graft with exclusion of the non-stent part, and the aortic arch. (D) Final schema of TENSE technique. TENSE, Total Exclusion of the Non-Stent part of the Frozenix J-graft using an Everting anastomosis.
Follow up
Forty-two patients underwent CTA before discharge, excluding one patient who died after aortic arch aneurysm rupture and one who died of interstitial pneumonia. After excluding these two patients who died during hospitalization, 42 patients were followed up on an outpatient basis every 6 to 12 months. Followed-up patients were censored at the point of the last known date that they had visited the hospital or based on the patient referral documents from the other hospital. The follow-up rate was 100%. The median follow-up duration was 1.9 years [interquartile range (IQR), 1.1–2.9 years].
Statistical analysis
Data were analyzed using EZR software, version 1.52 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is based on a modified version of R Commander (version 2.7-1) (R Foundation for Statistical Computing, Vienna, Austria). Numerical variables are expressed as median (IQR) or mean ± standard deviation, and categorical variables are expressed as numbers. Categorical variables were analyzed using Fisher’s exact test. The overall actual survival rate and major adverse cardiovascular or cerebrovascular event rates after discharge are expressed by Kaplan-Meier estimates. Statistical significance was set at P<0.05.
Results
Preoperative and intraoperative data
The patients’ preoperative characteristics are summarized in Table 1. The mean age of the patients was 75.0±6.8 years [median (IQR), 76.5 (69.8–81.0) years], and the patients comprised 35 men and 9 women. TENSE was performed for aortic arch aneurysms in 39 patients and for dissecting aneurysms in 5 patients (type A, n=3; type B, n=2). Three patients were diagnosed with aortic arch aneurysms when they developed hoarseness caused by left recurrent laryngeal nerve palsy, and one patient was diagnosed after developing hemoptysis caused by an aortobronchial fistula. Fifteen patients had existing cerebrovascular diseases that had been identified >6 months prior. Severe atherosclerosis with rich plaques was identified in nine patients. Three patients had previously undergone cardiothoracic surgery via median sternotomy. Among them, one patient with a distal aortic arch aneurysm had undergone aortic mechanical valve replacement and ascending aortic replacement. This patient developed a nonfunctioning mechanical aortic valve that required urgent repeat aortic valve replacement. Another two patients had previously undergone off-pump coronary artery bypass grafting as well as ascending aortic replacement for acute type A aortic dissection. We performed spinal cord drainage to prevent spinal cord complications in one patient who underwent thoracic endovascular repair preoperatively. The predicted mortality and morbidity rates were 10.0% and 40.2%, respectively, according to the JapanSCORE II.
Table 1. Patients’ preoperative characteristics.
| Variables | No. of patients (%) or median [IQR], N=44 |
|---|---|
| Age, yrs. | 76.5 [69.8–81.0] |
| Sex, female/male | 9 (20.5)/35 (79.5) |
| Body surface area, m2 | 1.66 (1.50–1.74) |
| Hypertension | 43 (97.7) |
| Dyslipidemia | 21 (47.7) |
| Diabetes mellitus | 14 (31.8) |
| Smoking | 34 (77.3) |
| Chronic kidney disease | 15 (34.1) |
| Hemodialysis | 2 (4.5) |
| Respiratory dysfunction | 7 (15.9) |
| Cerebral disease | 15 (34.1) |
| Extrathoracic vascular disease | 15 (34.1) |
| Atrial fibrillation | 3 (6.8) |
| Aortobronchial fistula | 1 (2.3) |
| RLNP | 3 (6.8) |
| Preop. left ventricular ejection fraction, % | 60.0 [57.0–60.3] |
| Vital shock requiring CPA | 1 (2.3) |
| Repeat surgery | 3 (6.8) |
| EuroSCORE II, % | 5.1 [3.0–8.8] |
| JapanSCORE II: mortality, % | 10.0 [7.0–16.5] |
| JapanSCORE II: morbidity, % | 40.2 [27.8–48.3] |
Categorical data are presented as number (%) of patients, and continuous data are presented as median (IQR). IQR, interquartile range; RLNP, recurrent laryngeal nerve palsy; CPA, cardiopulmonary arrest.
The patients’ perioperative data are summarized in Table 2. The mean open distal time was 40 minutes. Three patients with saccular distal aortic arch aneurysms underwent upper partial sternotomy. More than 50% of the patients underwent simultaneous operations. Seven patients underwent bicaval venous cannulation due to mitral valve, tricuspid valve, and atrial septal operations. Seventeen patients underwent coronary artery bypass, and five patients underwent aortic valve replacement with a bioprosthetic valve. We performed the modified Bentall procedure with a bioprosthetic valve in three patients and remodeling with aortic valve repair in one patient.
Table 2. Patients’ perioperative data.
| Variables | No. of patients (%) or median [IQR], N=44 |
|---|---|
| Operation time, min | 380 [323–453] |
| Cardiopulmonary bypass time, min | 196 [174–242] |
| Aortic clamp time, min | 137 [108–170] |
| Antegrade cerebral perfusion time, min | 133 [105–156] |
| Open distal time, min | 40 [37–50] |
| Frozenix J-graft size | |
| Diameter | |
| 27 mm | 2 (4.5) |
| 29 mm | 2 (4.5) |
| 31 mm | 8 (18.2) |
| 33 mm | 10 (22.7) |
| 35 mm | 18 (40.9) |
| 37 mm | 4 (9.1) |
| Stent-part length | |
| 90 mm | 16 (36.4) |
| 120 mm | 21 (47.7) |
| 150 mm | 7 (15.9) |
| Simultaneous procedures | |
| Aortic valve repair/replacement | 1 (2.3)/5 (11.4) |
| Bentall/remodeling procedure | 3 (6.8)/1 (2.3) |
| Mitral valve repair | 7 (15.9) |
| Tricuspid valve repair | 3 (6.8) |
| Coronary artery bypass grafting | 17 (38.6) |
| Atrial septal defect closure | 1 (2.3) |
| Upper sternotomy approach | 3 (6.8) |
| Transfusion | |
| Red blood cell, units | 8 [6–10] |
| Fresh frozen plasma, units | 8 [6–10] |
| Platelet, units | 20 [20–20] |
| Using intra-aortic balloon pumping | 1 (2.3) |
| Mortality | 2 (4.5) |
| Morbidity | 15 (34.1) |
| Postop. re-exploration for bleeding | 2 (4.5) |
| Postop. cardiac tamponade | 3 (6.8) |
| Postop. myocardial infarction | 1 (2.3) |
| Postop. lethal arrhythmia | 1 (2.3) |
| Postop. acute renal failure | 8 (18.2) |
| Postop. need for continuous hemodialysis | 5 (11.4) |
| Postop. cerebral accident | 4 (9.1) |
| Postop. spinal cord injury | 0 (0) |
| Postop. new RLNP | 1 (2.3) |
| Postop. prolonged mechanical ventilation (>72 h) | 4 (9.1) |
| Postop. tracheotomy | 2 (4.5) |
| Postop. pneumonia | 6 (13.6) |
| Postop. deep sternal wound infection | 1 (2.3) |
| Postop. intestinal disease | 2 (4.5) |
Categorical data are presented as number (%) of patients, and continuous data are presented as median (IQR). IQR, interquartile range; RLNP, recurrent laryngeal nerve palsy.
Postoperative results
Two patients (4.5%) died during hospitalization; one patient with a ruptured aortic aneurysm died of multiple organ failure caused by preoperative hemodynamic instability on postoperative day 2, although the aneurysm had been well excluded by the Frozenix, and the other patient died of interstitial pneumonia on postoperative day 27. Fifteen patients (34.1%) developed severe morbidity during hospitalization as follows: two patients required re-exploration for bleeding, one patient required transcatheter intervention of the left circumflex coronary artery for acute myocardial infarction, five patients required continuous hemodialysis, one patient developed a deep sternal wound infection but was cured by omental flap plombage after extensive debridement and negative-pressure wound therapy, and one patient developed new left recurrent laryngeal nerve palsy but was cured with time. No patients developed postoperative paraplegia or paraparesis, although four patients developed cerebral infarction {three patients intraoperatively [modified Rankin scale score of 1 in two patients and 4 in one patient (preoperative modified Rankin scale score of 3)] and one patient on postoperative day 7 (modified Rankin scale score of 4)}. All patients who developed postoperative cerebral infarction had an old cerebral infarction preoperatively (P=0.010). Among them, two patients demonstrated a shaggy aorta on preoperative CTA, but no significant relationship was observed between shaggy aorta and cerebral infarction (P=0.180).
In patients with aortic arch aneurysms, postoperative CTA showed no kinking of the Frozenix J-graft or proximal endoleaks. With the exception of two patients with residual aneurysms of the descending aorta that were scheduled to undergo secondary intervention, all aortic arch aneurysms were completely covered by the Frozenix J-graft. In patients with chronic aortic dissecting aneurysms, postoperative CTA showed no new entry (distal stent graft-induced new entry [dSINE]) into the descending aorta or kinking of the Frozenix J-graft. All patients with chronic dissection achieved complete thrombosis of the false lumen or exclusion of the dissected aorta.
Follow-up results
We followed up 42 patients as outpatients after discharge. We performed additional endovascular interventions for residual descending aortic aneurysms in two patients. The 1-, 2-, and 3-year postoperative overall survival rates were 95.5% [95% confidence interval (CI): 0.830–0.988], 91.6% (95% CI: 0.753–0.973), and 84.6% (95% CI: 0.598–0.947), respectively (Figure 2). Two patients died of ruptured type B aortic dissection and cerebral bleeding, respectively. In one patient with a ruptured type B aortic dissection after 3 years postoperatively, follow-up CT showed a gradual change in the angle of the open stent graft to a wide angle, which may have led to the type B aortic dissection, although there was some distance between the entry site of the type B dissection and the distal end of the open stent graft (Figure 3). However, the patient’s family was reluctant to pursue additional intervention for the ruptured type B aortic dissection because of the patient’s advanced dementia. Follow-up CT revealed aneurysmal shrinkage or no dilatation. The 1-, 2-, and 3-year major adverse cardiovascular and cerebrovascular event rates after discharge were 95.2% (95% CI: 0.823–0.988), 91.1% (95% CI: 0.738–0.972), and 83.5% (95% CI: 0.573–0.943), respectively (Figure 4). Four patients developed major adverse cardiovascular and cerebrovascular events, including death, during follow-up. Two patients died, and one patient underwent endovascular repair of a left common carotid artery aneurysm induced by an intraoperative tourniquet injury 4 months after surgery. One patient developed acute type B aortic dissection that did not require additional endovascular repair, but it was not associated with dSINE because of the long distance from the entry of the aortic dissection to the distal end of the open stent graft. With the exception of one patient with a ruptured type B aortic dissection, no patients demonstrated a distal change of the Frozenix J-graft.
Figure 2.

Estimated overall survival rate.
Figure 3.
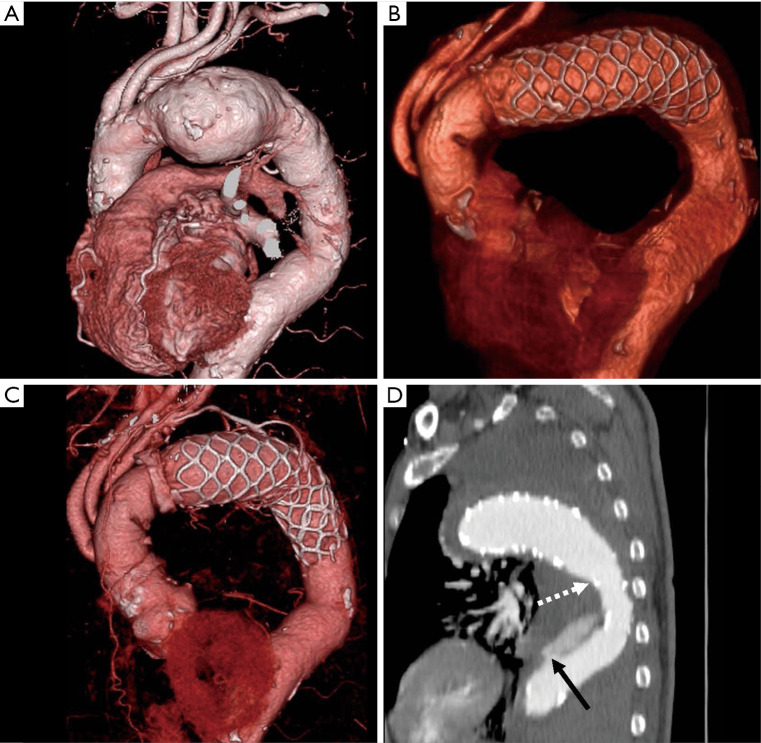
Computed tomography angiography image of a patient with ruptured type B aortic dissection. (A) Preoperative three-dimensional image. (B) Postoperative three-dimensional image. (C) Three-dimensional image and (D) sagittal image 3 years postoperatively. The black arrow shows the entry of the descending aortic dissection. The white arrow shows the distal end of the Frozenix J-graft.
Figure 4.
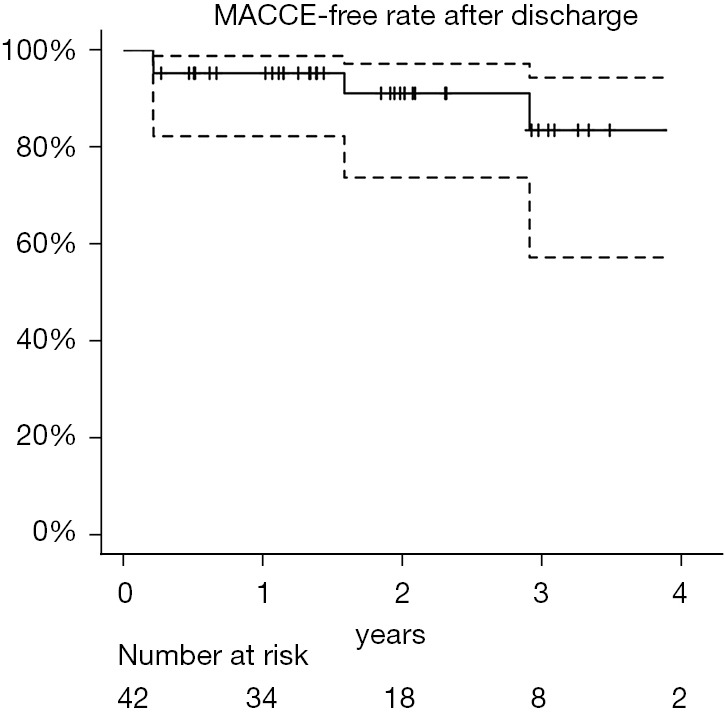
MACCE-free rate after discharge. MACCE, major adverse cardiovascular and cerebrovascular event.
Discussion
The FET technique is useful for the treatment of extensive aortic arch aneurysms or aortic dissection because it decreases the operation time and reduces the incidence of complications compared with conventional aortic arch replacement. The FET technique for aortic arch aneurysms that require zone 0 or 1 landing may be preferable to endovascular repair because of the high possibility of type 1a endoleaks and cerebral infarction. TAR with the FET technique for aortic arch aneurysms recently provided favorable outcomes with low mortality and morbidity rates compared with debranching thoracic endovascular repair (11). However, the FET technique is associated with some complications, including coarctation or kinking of the open stent graft and spinal cord complications. These complications may require additional interventions (1-4,6-8). Furthermore, left recurrent laryngeal nerve palsy sometimes occurs in TAR because of manipulation of the arch anastomosis distal to the left subclavian artery (1,2,4). Therefore, we designed the TENSE technique to prevent adverse events associated with the Frozenix J-graft with inclusion of an easily performed anastomosis technique. TENSE for aortic arch disease shows good early outcomes with a low mortality rate and no spinal cord complications, although high mortality and morbidity rates have been observed based on the JapanSCORE.
The Frozenix J-graft, which is commercially used in Japan, comprises non-stent and stent parts; this design differs from other open stent grafts used in Western Europe. Uchida et al. (1) reported kinking of the Frozenix J-graft in 1 of 60 patients. We previously reported two cases of kinking of the Frozenix J-graft after TAR with the FET technique for acute type A aortic dissection (6). Kinking of the Frozenix J-graft occurs mainly between the non-stent and stent parts for the following three reasons. First, the remaining long length of the non-stent part is positioned in the lesser curvature, and the short length of the non-stent part is positioned in the greater curvature of the aortic arch. Second, traction on the non-stent part may induce kinking. Third, distal migration of the stent part may occur. Uchida et al. (1) recommended keeping the non-stent part as short as possible to prevent kinking. Furthermore, a severely angulated arch is strongly associated with kinking. The translocation technique with TAR, in which the curved aortic arch is preserved, may also cause kinking, especially in young patients who develop acute aortic dissection with a severely angulated arch. However, the arch translocation technique is advantageous in that it allows for easy distal anastomosis and hemostasis of the anastomosis site because of the relatively shallower site of the anastomosis compared with conventional TAR. Therefore, considering the advantages and disadvantages of translocated TAR, we designed the arch translocation technique with total removal of the non-stent part of the Frozenix J-graft. Furthermore, after deploying the Frozenix J-graft, the non-stent part is commonly distorted into folds and wrinkles, which increases the difficulty of vascular graft anastomosis. Once modified, the Frozenix J-graft allows easy anastomosis with a wide opening in the stent part without the folded non-stent part.
Spinal cord complications significantly decrease patients’ activities of daily living. Recent reports have shown that after the FET technique, one risk factor for spinal cord complications is deployment of an open stent graft lower than the level of T8 to T10 (7,8). We experienced no cases of spinal cord complications because we did not deploy open stent grafts distal to T8. Moreover, we planned to perform secondary interventions in patients who required deployment of an open stent graft distal to T8 for an extensive aortic aneurysm. Etz et al. (12) showed that second-stage operations for extensive aortic aneurysms decrease the incidence of spinal cord complications. Therefore, for extensive aortic aneurysms requiring deployment of an open stent graft at a level lower than T8, second-stage treatment with the FET technique and additional endovascular completion may be necessary. Moreover, other risk factors, such as a low hemoglobin concentration, a low mean blood pressure, and a long open distal time (>60 minutes), are also associated with spinal cord complications (7). We performed distal arch anastomosis with a shorter open distal time (40 minutes), followed by management of patients with a relatively high mean arterial pressure and hemoglobin concentration of ≥10 g/dL, which may have prevented spinal cord complications. Maximum possible effort may be important to prevent spinal cord complications.
Stroke is a major complication that also associated with a decrease in patients’ activities of daily living after thoracic aortic surgery. In the FET technique with antegrade cerebral perfusion for aortic arch aneurysms, a stroke rate of 5.0% to 13.4% has been reported (1-3,7). We also experienced four patients (9%) who developed a cerebral infarction postoperatively, which is similar to previous data. Furthermore, our results showed that a previous cerebral infarction was significantly associated with postoperative cerebral infarction. This finding may suggest the presence of inherent factors that contribute to the development of cerebral infarction postoperatively even when strict preventive measures are taken.
Recurrent laryngeal nerve palsy is also highly associated with respiratory complications, especially in older patients. Some studies showed that the incidence of recurrent laryngeal nerve palsy was 0% to 18% after TAR with the FET technique for aortic arch disease (1,2,4). We encountered only one new recurrent laryngeal nerve palsy event in our study; however, this patient recovered with time. The reason may relate to the distal aortic arch anastomosis between the left common carotid and left subclavian arteries located proximal to the left recurrent laryngeal nerve when performing translocated TAR.
A minimal incisional approach may be an acceptable alternative to reduce postoperative complications, and it also has cosmetic advantages (13,14). El-Sayed Ahmad et al. (14) suggested that TAR with the FET technique via partial upper sternotomy can be performed safely and repeatedly in patients with extended thoracic aortic aneurysms. We also performed TAR with the FET technique via partial upper sternotomy in three patients. Translocated TAR with TENSE could be also applied using a minimal incisional approach because of easy distal anastomosis with minor bleeding from the anastomosis.
In patients with extensive dissecting aortic aneurysms, using the FET technique alone to close the proximal entry tear may not be sufficient because of reperfusion from residual distal re-entry tears that are left uncovered. Pacini et al. (15) showed that when using the FET technique to treat patients with chronic dissecting aneurysms, 79% of patients developed complete false lumen thrombosis, whereas 25% of patients required secondary aortic repair of the distal aorta because of reperfusion from distal re-entry. With endovascular repair for chronic type B aortic dissection, the most frequent cause of re-intervention is aneurysmal dilatation caused by reperfusion from distal re-entry tears left uncovered or newly created, or from visceral branch vessels (16). Therefore, these findings suggest that the main objective of intervention for type B dissecting aortic aneurysms should be to promote false-lumen thrombosis or to exclude the false lumen completely. In our study, all patients obtained good outcomes with complete thrombosis of the false lumen in the descending aorta because the dissection was located either at the aortic arch or at the proximal descending aorta. If the patients had developed extensive dissection to the thoracoabdominal aorta, additional interventions may have been required. Additionally, the FET technique offers an ideal proximal landing zone for endovascular repair compared with the conventional elephant trunk technique (17). Therefore, the FET technique may be effective and feasible in patients who require additional endovascular completion.
The deployment site of an open stent graft has the potential to cause dSINE (18,19). Spring-back force, which occurs when the stent graft has an inherent tendency to spring back to its initial position when passively bent at the curved aortic arch, has recently been noted (20). A recent case report showed that the spring-back force induced an aneurysmal change at the distal end of the open stent graft with a 60-mm stent graft (18). Moreover, in type B aortic dissection, a shorter stent graft length was significantly associated with dSINE, which raised speculation that shorter stent grafts have a stronger spring-back force (20). However, we experienced one patient who had a possibility of dSINE despite the fact that we deployed the stent part of the Frozenix J-graft in the straight zone of the descending aorta with a length of 120 mm. dSINE may have been derived from the relatively short length of the stent part in the straight zone of the descending aorta distal to the curved aortic arch, which is similar to short open stent grafts, and weakness of the aortic wall. Kreibich et al. (19) reported that the Thoraflex graft with its outer stent is associated with a high incidence (>20%) of dSINE because of its stiff outer distal ring compared with E-vita OPEN PLUS. In contrast, the Frozenix J-graft has an inner stent made of nitinol wire with a soft woven graft, which may reduce injury to the intima (1). Nevertheless, continuous follow-up is needed to detect adverse events that require additional intervention because dSINE can occur, even in the long term.
This study had two limitations. First, the study was a small retrospective case series. Second, we performed TENSE in only highly selected patients; however, we used this technique in patients with severely complicated conditions without technical issues and obtained low mortality and morbidity rates. Given these limitations, larger studies are needed to validate our observations.
Conclusions
In this study, our strategy provided relatively good early outcomes without spinal cord complications or Frozenix J-graft kinking in patients with aortic arch disease, despite a high JapanSCORE. TENSE prevented kinking of the Frozenix J-graft and allowed for easy distal anastomosis in translocated TAR with the FET technique. However, continuous follow-up is needed to avoid missing distal changes of the Frozenix J-graft.
Supplementary
The article’s supplementary files as
Acknowledgments
We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Ethics board of Osaka City Medical School Hospital (Approval No. 4203), and written informed consent was obtained from all the patients.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1751/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1751/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1751/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1751/coif). The authors have no conflicts of interest to declare.
References
- 1.Uchida N, Katayama A, Higashiue S, et al. A new device as an open stent graft for extended aortic repair: A multicentre early experience in Japan. Eur J Cardiothorac Surg 2016;49:1270-8. 10.1093/ejcts/ezv310 [DOI] [PubMed] [Google Scholar]
- 2.Chu MWA, Losenno KL, Dubois LA, et al. Early clinical outcomes of hybrid arch frozen elephant trunk repair with the Thoraflex hybrid graft. Ann Thorac Surg 2019;107:47-53. 10.1016/j.athoracsur.2018.07.091 [DOI] [PubMed] [Google Scholar]
- 3.Shrestha M, Martens A, Kaufeld T, et al. Single-centre experience with the frozen elephant trunk technique in 251 patients over 15 years. Eur J Cardiothorac Surg 2017;52:858-66. 10.1093/ejcts/ezx218 [DOI] [PubMed] [Google Scholar]
- 4.Shrestha M, Haverich A, Martens A. Total aortic arch replacement with the frozen elephant trunk procedure in acute DeBakey type I aortic dissections. Eur J Cardiothorac Surg 2017;51:i29-34. 10.1093/ejcts/ezw341 [DOI] [PubMed] [Google Scholar]
- 5.Weiss G, Santer D, Dumfarth J, et al. Evaluation of the downstream aorta after frozen elephant trunk repair for aortic dissections in terms of diameter and false lumen status. Eur J Cardiothorac Surg 2016;49:118-24. 10.1093/ejcts/ezv044 [DOI] [PubMed] [Google Scholar]
- 6.Morisaki A, Isomura T, Fukada Y, et al. Kinking of an open stent graft after total arch replacement with the frozen elephant technique for acute type A aortic dissection. Interact Cardiovasc Thorac Surg 2018;26:875-7. 10.1093/icvts/ivx387 [DOI] [PubMed] [Google Scholar]
- 7.Leontyev S, Tsagakis K, Pacini D, et al. Impact of clinical factors and surgical techniques on early outcome of patients treated with frozen elephant trunk technique by using EVITA open stent-graft: Results of a multicentre study. Eur J Cardiothorac Surg 2016;49:660-6. 10.1093/ejcts/ezv150 [DOI] [PubMed] [Google Scholar]
- 8.Flores J, Kunihara T, Shiiya N, et al. Extensive deployment of the stented elephant trunk is associated with an increased risk of spinal cord injury. J Thorac Cardiovasc Surg 2006;131:336-42. 10.1016/j.jtcvs.2005.09.050 [DOI] [PubMed] [Google Scholar]
- 9.Motomura N, Miyata H, Tsukihara H, et al. Japan Cardiovascular Surgery Database Organization . Risk model of thoracic aortic surgery in 4707 cases from a nationwide single-race population through a web-based data entry system: The first report of 30-day and 30-day operative outcome risk models for thoracic aortic surgery. Circulation 2008;118:S153-9. 10.1161/CIRCULATIONAHA.107.756684 [DOI] [PubMed] [Google Scholar]
- 10.Tamura N, Komiya T, Sakaguchi G, et al. ‘Turn-up’ anastomotic technique for acute aortic dissection. Eur J Cardiothorac Surg 2007;31:548-9. 10.1016/j.ejcts.2006.11.059 [DOI] [PubMed] [Google Scholar]
- 11.Bozso SJ, White A, Nagendran J, et al. Hybrid aortic arch and frozen elephant trunk reconstruction: Bridging the gap between conventional and total endovascular arch repair. Expert Rev Cardiovasc Ther 2018;16:209-17. 10.1080/14779072.2018.1429913 [DOI] [PubMed] [Google Scholar]
- 12.Etz CD, Zoli S, Mueller CS, et al. Staged repair significantly reduces paraplegia rate after extensive thoracoabdominal aortic aneurysm repair. J Thorac Cardiovasc Surg 2010;139:1464-72. 10.1016/j.jtcvs.2010.02.037 [DOI] [PubMed] [Google Scholar]
- 13.Deschka H, Erler S, Machner M, et al. Surgery of the ascending aorta, root remodelling and aortic arch surgery with circulatory arrest through partial upper sternotomy: Results of 50 consecutive cases. Eur J Cardiothorac Surg 2013;43:580-4. 10.1093/ejcts/ezs341 [DOI] [PubMed] [Google Scholar]
- 14.El-Sayed Ahmad A, Risteski P, Papadopoulos N, et al. Minimally invasive approach for aortic arch surgery employing the frozen elephant trunk technique. Eur J Cardiothorac Surg 2016;50:140-4. 10.1093/ejcts/ezv484 [DOI] [PubMed] [Google Scholar]
- 15.Pacini D, Tsagakis K, Jakob H, et al. The frozen elephant trunk for the treatment of chronic dissection of the thoracic aorta: A multicenter experience. Ann Thorac Surg 2011;92:1663-70. 10.1016/j.athoracsur.2011.06.027 [DOI] [PubMed] [Google Scholar]
- 16.Boufi M, Patterson BO, Loundou AD, et al. Endovascular versus open repair for chronic type B dissection treatment: A meta-analysis. Ann Thorac Surg 2019;107:1559-70. 10.1016/j.athoracsur.2018.10.045 [DOI] [PubMed] [Google Scholar]
- 17.Preventza O, Coselli JS, Mayor J, et al. The stent is not to blame: Lessons learned with a simplified US version of the frozen elephant trunk. Ann Thorac Surg 2017;104:1456-63. 10.1016/j.athoracsur.2017.03.072 [DOI] [PubMed] [Google Scholar]
- 18.Takagaki M, Midorikawa H, Yamaguchi H, et al. Rapidly progressed distal arch aneurysm with distal open stent graft-induced new entry caused by “spring-back” force. Ann Vasc Dis 2020;13:343-6. 10.3400/avd.cr.20-00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreibich M, Bünte D, Berger T, et al. Distal stent graft-induced new entries after the frozen elephant trunk procedure. Ann Thorac Surg 2020;110:1271-9. 10.1016/j.athoracsur.2020.02.017 [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Wang LF, Ma WG, et al. Risk factors for distal stent graft-induced new entry following endovascular repair of type B aortic dissection. J Thorac Dis 2015;7:1907-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as