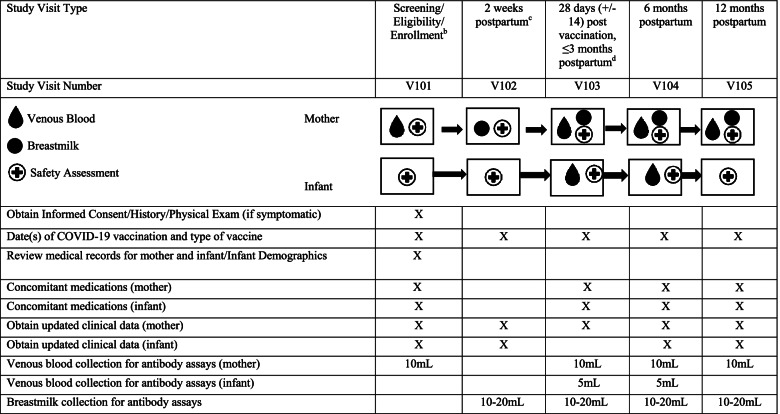
Table 2.
Schedule of Events for Multi-site Observational Maternal and Infant COVID-19 Vaccine Study (MOMI-Vax). Schedule of Events: Postpartum Women and their Infantsa
Abrreviations: mL milliliters
an = approximately 65 postpartum women who are scheduled to receive or have initiated any licensed or EUA COVID-19 vaccine series within first 2 months of delivery; n = approximately 65 infants of postpartum women
bFor mothers vaccinated prior to enrollment: Document COVID-19 vaccination date(s) and type
cOptional, for mothers enrolled at delivery
dIf visit overlaps with Visit 101 (i.e., mother received full series prior to enrollment), the two visits will be combined