Figure 3. Rheb regulates PDH activity independently of mTORC1.
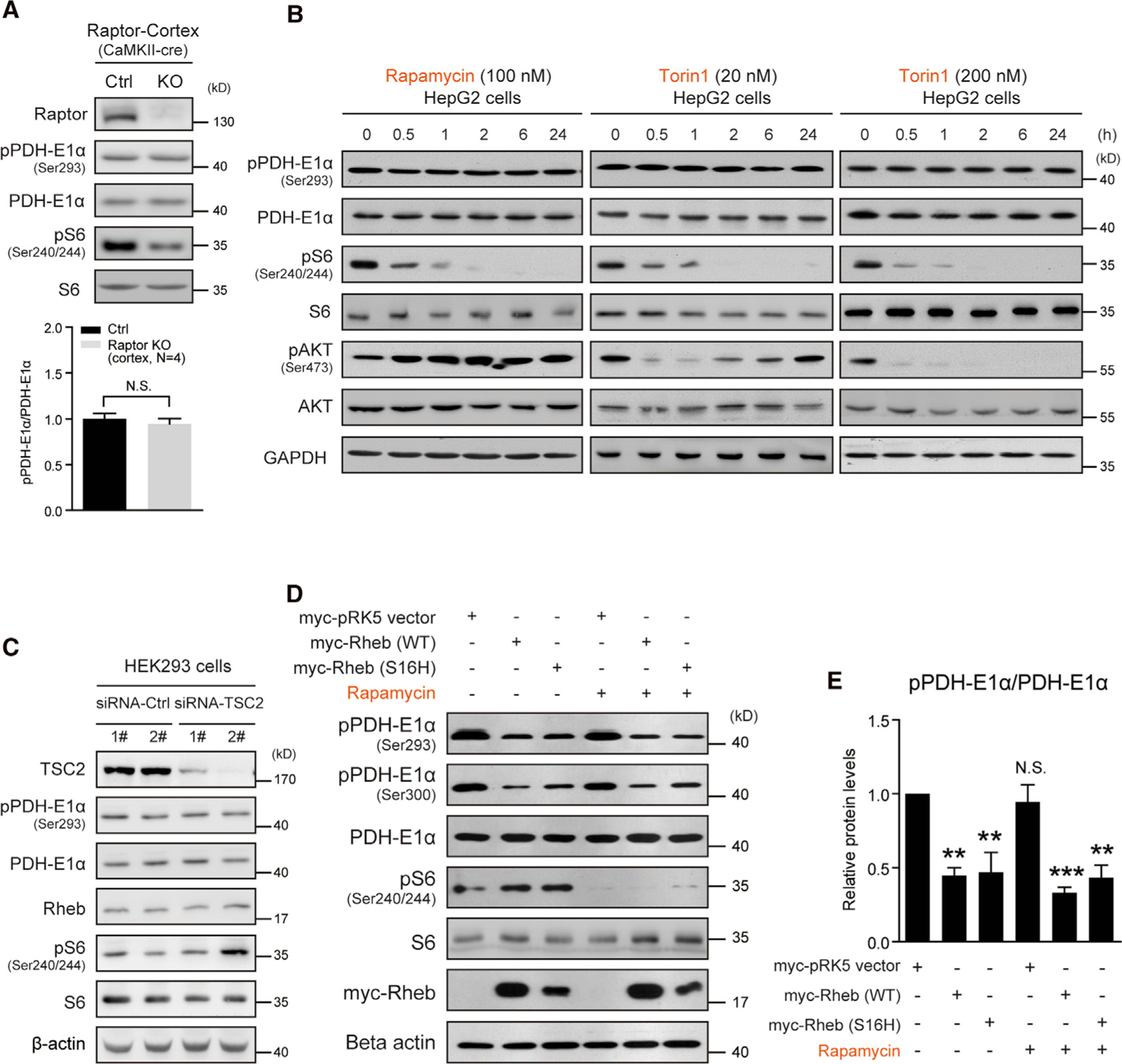
(A) Western blots and quantification indicating PDH phosphorylation not altered in the cortex of Raptor CaMKII-cre KO (Raptorf/f;CaMKII-cre) mice. Ctrl (raptorf/+ or raptorf/f). Ser293, cortex, N = 4 pairs of mice, p = 0.53.
(B) Western blots showing that rapamycin (100 nM) or Torin1 (20 and 200 nM) treatment for 24 h does not alter PDH phosphorylation in HepG2 cells. Note that mTORC1 activity was persistently inhibited, but mTORC2 activity assayed as pAKT (Ser473) initially decreased and then gradually recovered (Torin1 20 nM).
(C) Western blot showing that TSC2 knockdown activates mTORC1 activity, but does not alter PDH phosphorylation.
(D and E) Western blots (D) and quantification (E) showing that transient overexpression of Rheb (WT or S16H) decreases PDH phosphorylation in HepG2 cells and rapamycin (100 nM, 6 h) treatment does not prevent the decrease. pPDH-E1α (Ser293), N = 3 independent experiments, myc-pRK5 versus Rheb WT, p < 0.01; Rheb-S16H, p < 0.01; rapamycin, N.S.; Rheb WT + rapamycin, p < 0.001; Rheb-S16H+Rapamycin, p < 0.01. All data represent mean ± SEM. Statistical analysis was performed by using 2-tailed Student’s t test (A) or one-way ANOVA with Dunnett post hoc test (E), **p < 0.01 and ***p < 0.001. See also Figure S4.
