Abstract
Pentosan polysulfate (PPS) sodium (Elmiron) is the only Food and Drug Administration (FDA)–approved oral medication to treat interstitial cystitis (IC), also known as bladder pain syndrome. A symptomatic pigmentary maculopathy associated with PPS was reported in 2018. Since then, recognition of this unique drug toxicity has increased rapidly. This potentially sight-threatening side effect prompted the FDA in June 2020 to update the label for PPS to warn about “retinal pigmentary changes.” A challenging feature of pentosan maculopathy is its ability to mimic many other retinal conditions, including inherited retinal dystrophies such as pattern dystrophy, mitochondrially inherited diabetes and deafness, and Stargardt disease, as well as age-related macular degeneration (AMD). In this review we discuss the history of PPS maculopathy and its implications for thousands of at-risk IC patients. We use published literature and an illustrative case from our institution to highlight the importance of diagnosing PPS maculopathy. We also compare PPS maculopathy to AMD, explain why differentiating between the two is clinically important, and highlight avenues for further research. Finally, we highlight the paucity of data on patients of color and why this lack of understanding may impact patient care.
1. Introduction
In 2018, pentosan polysulfate (PPS), a drug to treat interstitial cystitis (IC), was described as a potential cause of a novel drug-associated pigmentary maculopathy.1 This sight-threatening and phenotypically heterogenous condition has important implications for management of IC and for patients who may have been misdiagnosed with similar-appearing ophthalmologic conditions. While pentosan maculopathy can resemble several inherited retinal disorders including pattern dystrophy, maternally inherited diabetes and deafness (MIDD), and Stargardt disease,2,3 the most prevalent condition PPS maculopathy mimics is age-related macular degeneration (AMD). Both PPS maculopathy and AMD manifest in the same region of the retina, have similar symptoms, and often begin in the sixth to seventh decades of life. Therefore, it may be difficult to differentiate between these diseases. In addition, when retinal toxicity does occur, it is uncertain whether stopping the drug stops progression of disease.4 Up to 1 million Americans may have IC5 and at least tens of thousands of people are at risk for pentosan maculopathy.6 In one of our patients, maculopathy presented after she stopped PPS and continued to progress for the next decade.
In this review we summarize the literature and frame PPS maculopathy as a new and insidious “masquerader” of AMD. Since the age of onset, signs, and symptoms of AMD and PPS maculopathy are similar, they may both be present in the same eye and could modify each other.7 It is also possible that PPS may cause AMD to manifest earlier than it otherwise might in patients who are genetically at risk for AMD. We review published studies and present an illustrative case from our institution to show how and why these 2 conditions can result in misdiagnoses in clinical practice. Distinguishing the 2 conditions is vital because the management of patients with these disorders is different and has implications for patients suffering from IC, a condition with significant quality of life impacts.8,9
Finally, we address some controversies regarding pentosan maculopathy, including lack of workable, real-world screening criteria. We also describe the paucity of data on this disease in people of color and in countries outside the US.
2. Background
2.1. Interstitial Cystitis
Interstitial cystitis (IC), also known as bladder pain syndrome, is an occasionally debilitating and often difficult to manage condition consisting of chronic bladder and pelvic pain along with urinary frequency and nocturia.4 Although little is currently known about the epidemiology of IC, approximately 450,000 to 700,000 Americans appear to be afflicted with this disorder,10 85% of whom are women.11 Most patients in reported studies lived in the US and were predominantly Caucasian.
IC has a high societal burden, costing an estimated $428 million in medical cost and sick leave in 1987, or over $1.2 billion today.12 The pathophysiology of IC is multifactorial. It involves a complex interplay between peripheral and central aberrations involving the bladder mucosa, inflammation, and central dysregulation of bladder sensitivity.11–13 This complexity has made finding effective treatments difficult.
First-line therapy is conservative management with lifestyle changes including diet and activity modification. Second-line treatment includes physical therapy and 4 oral agents as outlined by the 2014 American Urological Association: amitriptyline,14 hydroxyzine, cimetidine, and pentosan.16 Tricyclic antidepressants other than amitriptyline and antiepileptics have been tried, as well as intravesical therapy with bupivicaine, triamincolone, heparin, buffered lidocaine, dimethyl sulfoxide, or sodium oxychlorosene.13 Of these treatments, pentosan is the only oral agent approved by the Food and Drug Administration (FDA) for treatment of IC.
2.2. Pentosan
Pentosan polysulfate (PPS) sodium is a heparin and glycosaminoglycan-like anticoagulant and semisynthetic sulfated polysaccharide which has been used for the treatment of IC in an off-label fashion since the 1980s.16 After gaining FDA approval in 1996 and being marketed as Elmiron (Janssen Pharmaceuticals, Titusville, NJ), PPS has grown in popularity as a first- or second-line treatment for IC.15,17,18
PPS is believed to substitute for a deficiency in the glycosaminoglycan layer in the bladder urothelium of IC patients. It is usually given orally but can also be delivered through an intravesical route.15 A 2006 review of the available data on the efficacy of pentosan in treating IC concluded that PPS was beneficial.19
While more recent studies show no benefit over placebo,20,21 multiple patients at our institution have described the effect of the drug as ‘life-altering.” Some of our patients who have attempted to discontinue PPS or decrease their dose have a recurrence of painful flares. The reliance on this drug is one of the factors that makes pentosan maculopathy vexing for clinicians.
The most common nonocular side effects are nausea, diarrhea, and headache.19 Due to burgeoning awareness of the retinal toxicity, the FDA revised the label for pentosan to reflect this new side effect.22
3. Pentosan Maculopathy
3.1. Discovery
Pearce and coworkers identified a group of 6 patients who presented with a pigmentary maculopathy. These patients were negative for mitochondrial disease and macular dystrophies, and it was postulated their condition was secondary to pentosan.1 The evolving understanding of this condition will be discussed in the following sections.
3.2. Associations, Risk Factors, and Prevalence
There has been a growing recognition of PPS maculopathy among patients at multiple institutions. A question in this early phase was whether it was the drug or the underlying condition causing the retinal changes, or a combination of the 2. Following the initial case series, 4 studies performed at 4 different institutions helped determine the strength of association between pentosan and this novel maculopathy. In the first such study by Hanif et al, expert image graders masked to patient medication history correctly identified 14 cases of the condition in patients exposed to pentosan. None of the unexposed group were identified as having characteristic findings. Only PPS exposure, and no other IC therapy, was significantly associated with a pigmentary maculopathy.23 In 2020, Vora and coworkers found that 23 out of 117 patients exposed to at least 500 g of PPS showed signs of the maculopathy. Cumulative dose was the only significant risk factor and development of maculopathy varied in a dose-dependent manner.24
Wang and coworkers, in a prospective study of PPS users, found 10 out of 50 had signs of maculopathy. These patients also had significantly greater mean duration of exposure, daily dose, and cumulative dose.25 Finally, Higgins et al showed a significant dose-response relationship between PPS exposure and presence of maculopathy in 3 out of 96 PPS users at the University of California at Davis.26
Cumulative dose dependence of PPS with maculopathy has been seen with multiple studies: patients with PPS maculopathy used the drug for an average of 15.0 [±5.7 standard deviation (SD)] years, with an average cumulative exposure of 1824 (±1042 SD) g.27 So far it remains unclear whether there is a minimum safe cumulative dose, as 1 case of the disease presented in a 44-year-old woman who had taken only 435 g of pentosan total.28 It is also unclear to what extent cumulative dose per unit body weight affects the development of maculopathy, with 1 study showing a higher risk with a dose greater than 20g/kg.29 Daily dose may modulate risk as well. Wang and coworkers found that affected patients took a mean daily dose of 444.8 mg versus 301.8 mg for unaffected patients.25 In a study by Uner et al, 1 in 6 patients who had been on the drug for more than 15 years at 100 mg daily reported macular disease versus 6 of 10 with a 500 mg daily dose, despite being on the drug for an average of 5 years.30 It appears possible that there is a threshold daily dose for macular disease, though more work is needed to identify subclinical cases of maculopathy and identify other risk factors. Genetic predisposition to age-related macular degeneration (AMD) could be a risk factor for PPS maculopathy.
Estimates of the prevalence of PPS maculopathy vary. Wang et al reported an overall 20% rate in their cohort.25 In that study, among patients with 500 g to 999 g of exposure, 12.7% had maculopathy. Between 1000 g and 1,500 g that number increased to 30%, and above 1500 g the rate was 41.7%. Those with greater than 1500 g of exposure were nearly 5 times as likely to have PPS maculopathy, compared to those with 500g to 999 g cumulative exposure.25 Vora and coworkers reported a prevalence of 25%.24
3.3. Symptoms and Functional Impairment Are Similar to Age-Related Macular Degeneration
The most commonly reported symptoms of PPS maculopathy are prolonged dark adaptation, difficulty reading, metamorphopsia, nyctalopia, and occasionally scotoma, most often in the setting of preserved acuity. Pooled data from several studies show about 9% of patients are asymptomatic.27 The 6 patients from the initial series at Emory had a mean acuity of 20/20.1 The authors note the possibility of referral bias in these data given that symptomatic patients are more likely to seek care, and often are sent to tertiary care centers.
A study by Lyons and coworkers quantified functional impact on patients through a battery of tests. Patient responses to the National Eye Institute Visual Function Questionnaire-39 and the Low Luminance Questionnaire showed greater dysfunction than similar patients with intermediate age-related macular degeneration (AMD).31 Tests of dark adaptometry by Lyons et al showed that 6 patients with mild disease had rod-intercept times less than 6.5 minutes, the threshold for intermediate AMD for the device used (AdaptDx, Magulogix, Inc, Harrisburg, PA) while more severe cases had rod-intercept times of over 20 minutes.32
3.4. Clinical and Imaging Features
On exam, patients present with hyperpigmented macular spots, deep yellowish subretinal deposits, and patchy paracentral retinal pigment epithelium (RPE) atrophy in more advanced disease.1,25,33 Hanif and coworkers postulated that with advancing disease, the hyperpigmented spots disappeared and gave rise to RPE atrophy.33
The features on dilated fundoscopic examination are much more subtle than those found on other imaging modalities. The most striking features of this disease are revealed on fundus autofluorescence (FAF). Numerous studies have shown a densely packed array of hyper- and hypoautofluorescent spots centered around the fovea symmetrically between both eyes. Many eyes exhibit a peripapillary halo of hypoautofluorescence that contrasts to the sparing of FAF signal often seen in hereditary maculopathies.33 Hyperautofluorescent spots colocalize with darkly pigmented spots and yellowish subretinal deposits seen on color fundus photography.27 Hanif also showed using ultra-widefield autofluorescence (AF) imaging that disease was confined to the posterior pole in 56% of eyes and diffuse disease extended to the far retinal periphery in 36% of eyes.33
Optical coherence tomography (OCT) reveals hyperreflective RPE nodules which correspond to the macular pigment clumps seen on fundus photography. These nodules may not be present in advanced disease, when RPE atrophy occurs. 1,25,33 Some cases also show intraretinal hyperreflective foci like those seen in AMD that may represent dissociated RPE cells.26 Many cases show outer retinal irregularity, retinal thinning, and RPE atrophy.25,33
Investigators have also noted the possible importance of near-infrared reflectance (NIR) in screening for PPS maculopathy. NIR may be more sensitive in detecting preclinical disease since NIR abnormalities can exist despite a normal appearance on other imaging modalities with exception of FAF.27
However, with any new disease, the phenotypic descriptions are bound to be biased by their presentation at tertiary referral centers. As we learn more about the pathophysiology and presentation of PPS maculopathy, we will better define its phenotypic spectrum. This has vital implications for clinicians trying to diagnose new cases in everyday practice, and to tease out this condition from the others it mimics within their practice.
3.5. Mechanism of Toxicity
While the pathophysiology of pentosan maculopathy is still unknown, the clinical and imaging findings, along with the mechanism of action of pentosan, provide potential clues. Pentosan has a glycosaminoglycan-like backbone with highly negatively charged sulfate groups that may lead to nonspecific interactions with positively charged compounds. PPS has a number of anticoagulant, anti-inflammatory, prochondrogenic, and fibrinolytic properties.34,35 Greenlee et al proposed that long-term fibroblast growth factor (FGF) antagonism may be the mechanism of damage either directly to cells or in preventing cellular repair.36 PPS inhibits FGF signaling at the level of the RPE, and FGF in zebrafish is associated with photoreceptor maintenance.37
Given the similarity of PPS maculopathy to mitochondrial maculopathies, Yusuf et al proposed that PPS altered mitochondrial function through an unknown mechanism.38 Hanif and coworkers proposed 2 other possibilities based on the similarity of the structure of the composite glycosaminoglycans to PPS itself: direct toxicity to RPE which would inhibit processing of photoreceptor outer segments, or disruption of the interphotoreceptor matrix that mediates the photoreceptor–RPE relationship, especially since the insoluble components of the interphotoreceptor matrix are comprised of chondroitin sulfate proteoglycans.33,39 Findings of choriocapillaris dropout may implicate a vascular cause as is the case in early and late AMD.40–42
3.6. Complications and Progression
PPS maculopathy can also cause vision loss through progression of RPE atrophy, cystoid macular edema (CME), and/or choroidal neovascularization (CNV). 6,24,25,33 In the study by Hanif and coworkers, 39% of eyes had AF- and OCT-validated evidence of parafoveal RPE atrophy which coalesced and involved the fovea with increasing severity. They also found 9 eyes in 6 patients had cystoid macular edema with leakage on fluorescein angiography in 1 case.33 Wang et al found RPE atrophy in 50% of patients.25 The CME in these case series was successfully treated by standard-of-care therapies such as topical or oral carbonic anhydrase inhibitors, topical steroids, and intravitreal anti-vascular endothelial growth factor (VEGF) therapy.27,43
CNV has been reported in 2 published cases33,44 and was successfully managed with anti-VEGF injections. The case below from our institution is instructive in this regard because we believe it shows how easily AMD and PPS maculopathy can be conflated.
A troubling aspect of PPS maculopathy with important implications for practice is its tendency to progress after discontinuation of the drug. A case study from Huckfeldt and Vavvas describes a patient who progressed 6 years after stopping the drug. The patient in this report had visual acuity decrease from 20/20 on the right eye and 20/50 on the left eye to 20/80 on the right eye and 20/400 on the left eye with progressive central RPE atrophy seen on multimodal imaging.4 The possibility that pentosan maculopathy worsens after discontinuation reinforces the importance of early screening.
3.7. Severity Grading and Screening
There is no agreed-upon severity grading scale for the disease. Hanif et al used imaging findings to grade severity and stratified cases based on the extent of disease and the presence or absence of atrophy: mild cases were defined as being contained within the vascular arcades and lacking atrophy; moderate cases were defined as reaching but not extending more than 2 disc diameters beyond the temporal vascular arcades, or the presence of noncentral RPE atrophy; severe cases were defined either as disease extending greater than 2 disc diameters beyond the temporal vascular arcades or the presence of RPE atrophy involving the foveal center.33
Wang and coworkers used atrophy alone to determine severity. Mild cases had no atrophy, moderate cases had discrete areas of atrophy, and severe cases had diffuse atrophy.25 Both schemes have limitations: in Hanif and coworkers’ study, no association between disease severity and PPS exposure was found.33 Wang and coworker’s scale did find correlation between cumulative exposure and severity but was limited to only 20 patients, so statistical comparisons could not be made.25
There is no consensus regarding screening guidelines either, though some have been proposed.25 We suggest that patients new to pentosan should be counseled extensively on the risks and benefits of the medication and use the lowest dose required to control symptoms. Pentosan should be prescribed with extreme caution in patients with preexisting macular disease, especially AMD, given the potential for PPS to modify the phenotype of AMD.7 There should be clear communication between the eye doctor, prescribing physician, and patient about the presence of PPS maculopathy so that the best course can be agreed upon. There are also few effective, noninvasive alternative treatments for IC, unlike conditions treated with other retinotoxic medications, eg, rheumatoid arthritis and hydroxychloroquine.45 Some patients may choose to continue using pentosan despite evidence of retinal damage.
It is also prudent to screen patients at baseline then yearly with multimodal imaging (FAF, OCT, and/or photos) and once toxicity is detected, have another discussion about the risks, benefits, and alternatives to continuing PPS. Though the average duration of exposure is 10 years, yearly screening makes sense because symptomatic maculopathy has been detected in as little as 3 years and 435 g cumulative dose,28 and because the visual symptoms can be debilitating and unavoidable after a certain threshold. As a practical matter, it might be easy to lose patients to follow up with intervals beyond 1 year. Finally, treatable complications from PPS maculopathy —CME and CNV—may be undetected by the patient but picked up on clinical exam.
4. Pentosan Maculopathy as a Masquerader
It is apparent from studies of population data that PPS maculopathy patients have been misdiagnosed. In one such study, Uner et al found that 19.7% of patients exposed to more than 1031 g of PPS were more likely to have a diagnosis of AMD and/or a pigmentary maculopathy.30 A 2019 study of claims data from 2002 to 2016 showed that after 7 years, the 1604 PPS users screened were more likely to be diagnosed with nonexudative AMD or a pigmentary maculopathy compared to controls. No statistically significant association existed at 5 years of exposure.46
In the following sections we describe how pentosan maculopathy can present as several other disorders and the difficulty in distinguishing them. The case from our institution was very effective in mimicking AMD.
4.1. Pentosan Maculopathy and Inherited Retinal Dystrophies
Barnes and coworkers set out to determine if masked graders could differentiate PPS maculopathy from inherited retinal dystrophies (IRDs) based on fundus imaging alone.47 They identified 1131 patients with sufficient imaging who had been diagnosed with any of the following diagnosis codes: unspecified hereditary retinal dystrophy, pigmentary retinal dystrophy, other dystrophies primarily involving the sensory retina, dystrophies primarily involving the retinal pigment epithelium (RPE), or unspecified macular degeneration.47 Two graders masked to the patient’s medication history then reviewed imaging from each case and categorized the patients into 1 of 3 bins: highly suggestive of PPS maculopathy, some features resembling PPS maculopathy, and clearly distinct from PPS maculopathy, based on the following 6 criteria:47
Fundus photography revealing macular hyperpigmented spots, yellow-orange deposits, patchy RPE atrophy, or a combination thereof
FAF imaging revealing a densely packed array of hyperautofluorescent and hypoautofluorescent spots involving the posterior pole, centered on and involving the fovea
OCT imaging demonstrating focal thickening or elevation of the RPE with associated hyperreflectance on near-infrared reflectance imaging
A peripapillary hypoautofluorescent halo in patients showing involvement of the peripapillary retina (compared to ABCA4-associated Stargardt disease which has peripapillary sparing)
Hyperautofluorescent spots that were no larger than 2 venule widths (ie, major venules off optic disc), except in situations where 2 clearly distinct foci of hyperautofluorescence were connected in a reticular pattern, and
Absence of typical drusen (classic yellow-white lesion under the RPE)
The masked reviewers categorized 15 patients as highly suggestive of PPS maculopathy, 25 as showing some features but not classic disease, and 1091 as clearly distinct from PPS maculopathy. The unmasked reviewer found 10 patients had classic disease, and all these were correctly identified by the masked graders. Five patients were misclassified as having features highly suggestive of disease but in reality had no pentosan exposure. One of these had an ABCA4 variant.47 The unmasked grader reviewed these cases and found features in each case that differed from the criteria laid out above. The results of this study showed that the masked reviewers had a 100% sensitivity and 99.6% specificity in identifying pentosan maculopathy.47
The authors concluded that using the 6-point case definition, masked reviewers “were able to differentiate PPS maculopathy reliably from other pigmentary maculopathies.”47 They further stated that this study should reassure providers of their ability to diagnose PPS maculopathy through clinical exam and multimodal imaging.
There are several limitations to this study and application of this to real-world practice. The grading system, by the authors’ admission, is complex, unwieldy for rapid assessment and speaks to the high degree of similarity between these conditions. Also, among the 25 patients who were judged to show some features but not classic disease, none had pentosan exposure. Nine of these patients had molecularly confirmed inherited retinal disease. Of the remaining 16, 9 had a macular dystrophy, 4 had an indeterminate dystrophy, 1 had AMD, and 1 had a cone-rod dystrophy.47 Interestingly, their database search also identified 1 previously undiagnosed case of PPS maculopathy in 1 of their patients who had a remote history of pentosan exposure.
4.2. Pentosan Maculopathy and Age-Related Macular Degeneration
In a study similar to Barnes and coworkers,47 Christiansen et al evaluated the ease with which pentosan maculopathy could be distinguished from AMD. The authors identified 90 patients with IC who had been seen at the Emory Eye Center between 2014 and 2019 and had fundus imaging available for review. Masked reviewers then categorized these patients into 4 categories: 1) pentosan maculopathy, 2) AMD or drusen, 3) neither, or 4) unsure.7 Patients were determined to have PPS maculopathy based on the first 3 of the imaging grading criteria listed in the previous section Pentosan Maculopathy and Inherited Retinal Dystrophies.
The masked reviewers placed 17 patients in category 1, 25 in category 2, 47 in category 3, and 1 in category 4. Among categories 1 to 4, 17 (100%), 15 (60%), 28 (60%), and 0 patients had exposure to PPS (P = 0.007), respectively. Mean cumulative exposure to PPS across the 4 categories was 2.1 kg, 0.36 kg, 0.34 kg, and 0 kg, respectively (P < 0.00001).7
The results again showed high sensitivity to the detection of PPS maculopathy: 100% of the eyes placed in category 1 had exposure to PPS, with a mean cumulative dose of 2.1 kg. In addition, the AMD or drusen group was older (mean age 71.6 vs 59.8 years) and had less PPS exposure compared to the patients in category 1. A subsequent unmasked review showed that there were no typical macular drusen in any of the 17 patients in category 1, leading the authors to postulate that the absence of drusen might need to be added to the case definition of pentosan maculopathy. Interestingly, the sole patient in category 4 (unsure), had molecularly confirmed ABCA4 retinopathy.7
AMD and pentosan maculopathy are sufficiently distinct clinical entities that providers should be able to separate them based on clinical exam and multimodal retinal imaging.7 Perhaps the more relevant implications are based on an analysis of the patients in category 2 (AMD or drusen). Among the 25 patients in category 2, 15 had been exposed to PPS and among these, 8 had RPE pigment clumps and 3 had RPE atrophy. In the remaining 10 patients, the ones without PPS exposure, only 1 had RPE pigment clumps and 2 had RPE atrophy.7 These findings are consistent with clinical features of PPS maculopathy previously described and imply that pentosan alters the phenotype of AMD.7 The authors stated that given the small sample size, more study would be needed, but cautioned the use of pentosan in patients with preexisting macular disease.
4.3. Pentosan Maculopathy as an Age-Related Macular Degeneration Masquerade: A Case Study
Here we describe a case from our institution that exemplifies the difficulty in distinguishing AMD from PPS maculopathy in clinical practice along and its implications.
A 47-year-old woman was self-referred to our institution for continued management of neovascular AMD (nAMD) in the left eye and presented to our clinic in January 2013. Previously, she had received 4 ranibizumab injections in that eye starting February 2012 with the last one in May 2012. Her past medical history was significant for chronic interstitial cystitis, anxiety, and depression. Review of systems was noncontributory. She was a never-smoker and there was no family history of macular degeneration. She had taken PPS for 10 years at a dose of 200 mg twice a day for a cumulative dose of 1460 g and discontinued it at age 45. She complained of intermittent blurry vision, difficulty reading, and prolonged dark adaptation in both eyes (OU). Outside records revealed “slight pigment changes” and drusen in both maculae.
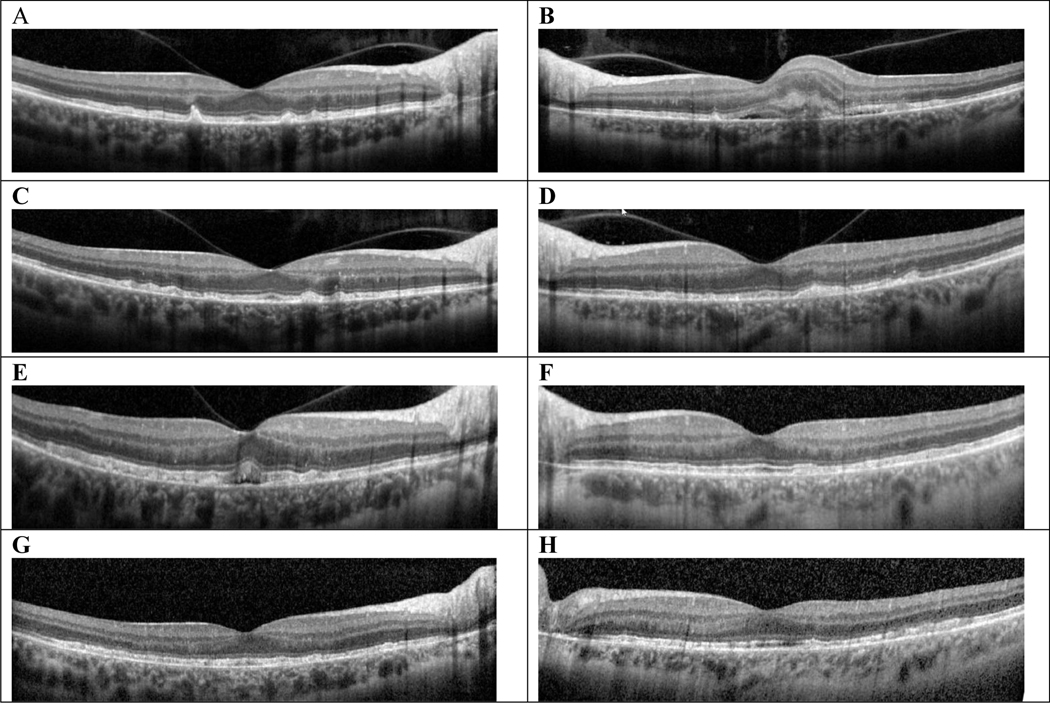
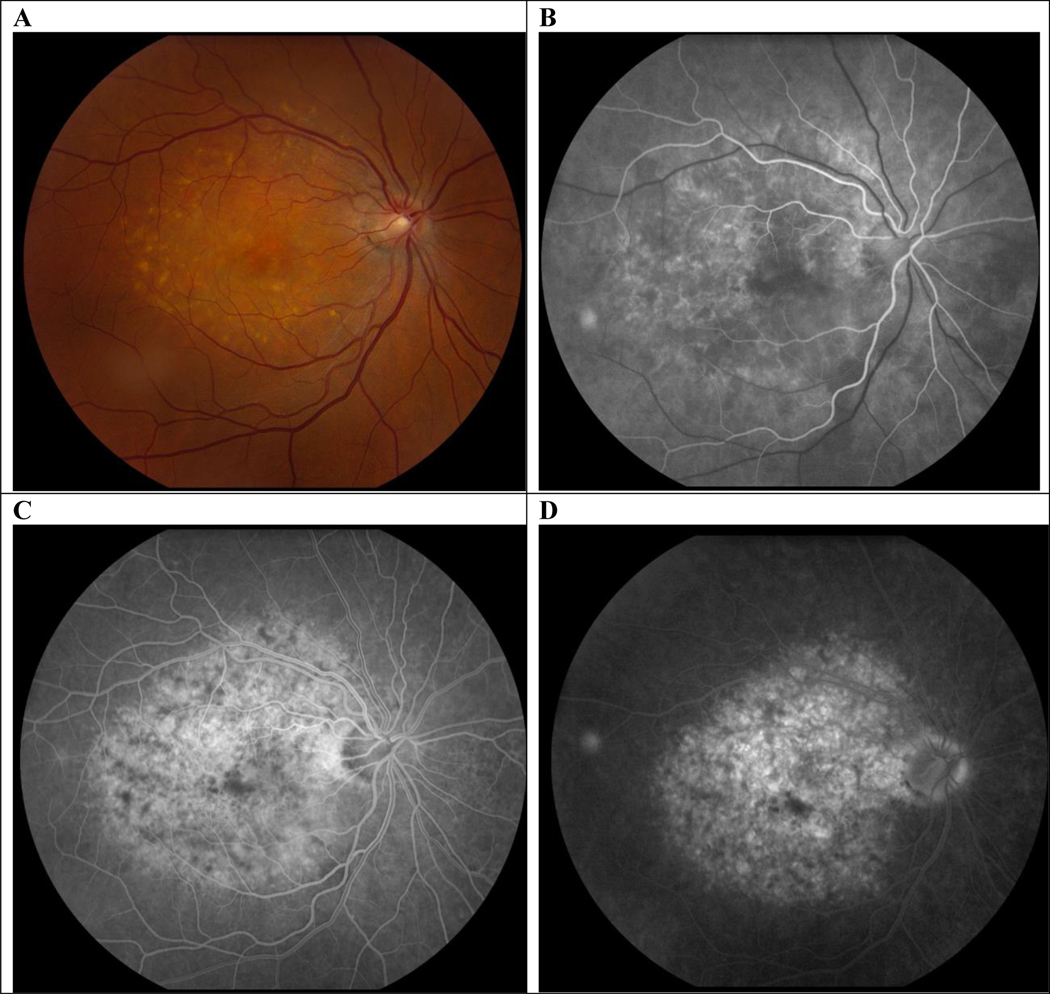
Her presenting complaint in our clinic was metamorphopsia and she was noted on her chart to have “numerous drusen” OU and a “choroidal neovascular membrane with subretinal hemorrhage” in the left eye (OS), along with a “small area of pigment clumping temporally.” Her visual acuity (VA) was 20/20 in the right eye (OD) and 20/150 OS, and the remainder of the exam was noncontributory. Fundus exam (Fig. 1) showed macular hyperpigmented spots and yellow-orange deposits OU which looked like soft drusen on biomicroscopy and a perifoveal hemorrhage OS. Fluorescein angiography (Fig. 1) showed blockage in the left eye from hemorrhage with surrounding late leakage, and the right eye had staining of subretinal deposits but no leakage. OCT (Fig. 2) demonstrated drusen and reticular pseudodrusen, aka subretinal drusenoid deposits48 OU. OCT OS showed fluid and outer retinal changes consistent with a choroidal neovascular membrane. A diagnosis of nAMD OS was given and she received an injection of intravitreal aflibercept in this eye.
Figure 1.
A and B, At presentation, color photos show macular hyperpigmented spots, yellow-orange deposits and patchy RPE atrophy OU and retinal hemorrhage OS. C and D, late frame of the fluorescein angiogram shows blockage from the hemorrhage OS with surrounding leakage. OS indicates left eye; OU, both eyes; RPE, retinal pigment epithelium.
Figure 2.
OCT stack with a horizontal cut through the foveal center at each time point showing evolution of both eyes. A and B, At initial visit, this 47-year-old woman had drusen and SDDs OU, and subretinal fluid with subretinal hyperreflective material OS consistent with CNV OS. C and D, 2 months after initial presentation, scattered RPE elevations and SDDs OU with regressed CNV OS. E and F, 2 years after initial presentation, there was new subretinal fluid OD, RPE/EZ irregularities, diffuse RPE elevations, EZ band irregularities OU. G and H, 8 years after presentation, diffuse RPE/EZ band irregularities. CNV indicates choroidal neovascularization; EZ indicates ellipsoid zone; OCT, optical coherence tomography; OD, right eye; OS, left eye; OU, both eyes; RPE, retinal pigment epithelium; SDD, subretinal drusenoid deposits.
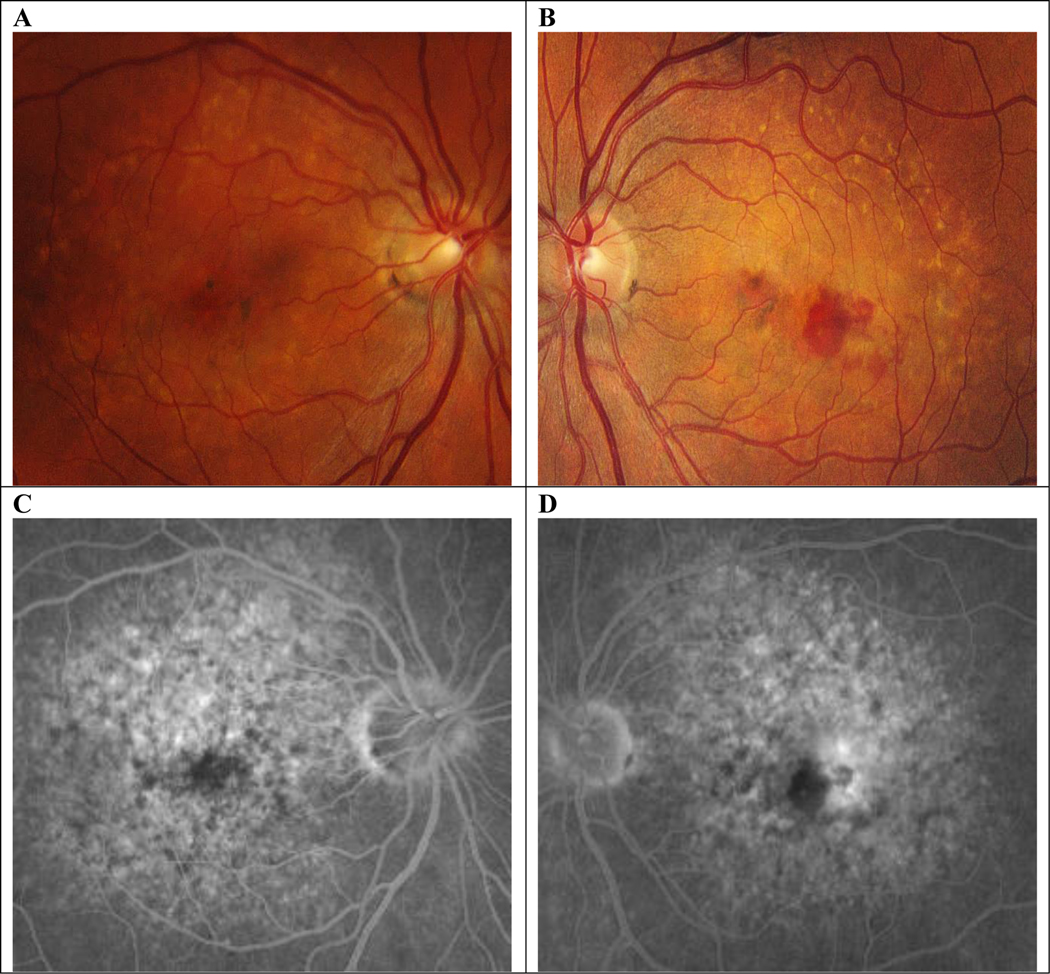
Six weeks later her VA OS improved to 20/40+2 and her subretinal fluid resolved. She received her second injection of aflibercept and continued to follow up with a treat-and-extend strategy until her treatment intervals were extended to every 12 weeks. She continued receiving intravitreal injections for the following 2 and a half years during which time her VA improved to 20/20 and remained stable. During this time (at the relatively young age of 50) she was noted to have fluid on OCT (Fig. 2E) and an area of leakage on FA OD (Fig. 3). Given the constellation of findings and clinical scenario, she was determined to have new conversion to nAMD OD and intravitreal anti-VEGF therapy was initiated. Her intra- and subretinal fluid had resolved by her follow-up in 4 weeks.
Figure 3.
A, Color fundus photo of right eye. B–D, early, mid, and late frames, respectively, of fluorescein angiogram showing mild-late leakage near the fovea.
Her OD was monitored over the next 14 weeks without treatment. She then had recurrent intraretinal fluid and aflibercept was resumed OD. Between December 2015 and September 2020, she had been treated with bilateral aflibercept injections every 8 to 16 weeks. Her OS remained dry during those visits but her OD had worsening intraretinal fluid at the shorter intervals. Figure 2 shows OCT scans at various timepoints showing evolution of her macula from presentation in 2013 until today.
In the summer of 2021, the patient asked if her condition could be due to prior exposure to PPS. Figure 4 shows autofluorescence imaging done in June 2021 consistent with the established case definition of pentosan maculopathy. On exam she had hyperpigmented spots and yellow-orange deposits on exam and a densely packed array of hypo- and hyperautofluorescent spots in the macula on FAF. OCT shows RPE elevations with some reticular pseudodrusen but no classic drusen consistent with AMD.
Figure 4.
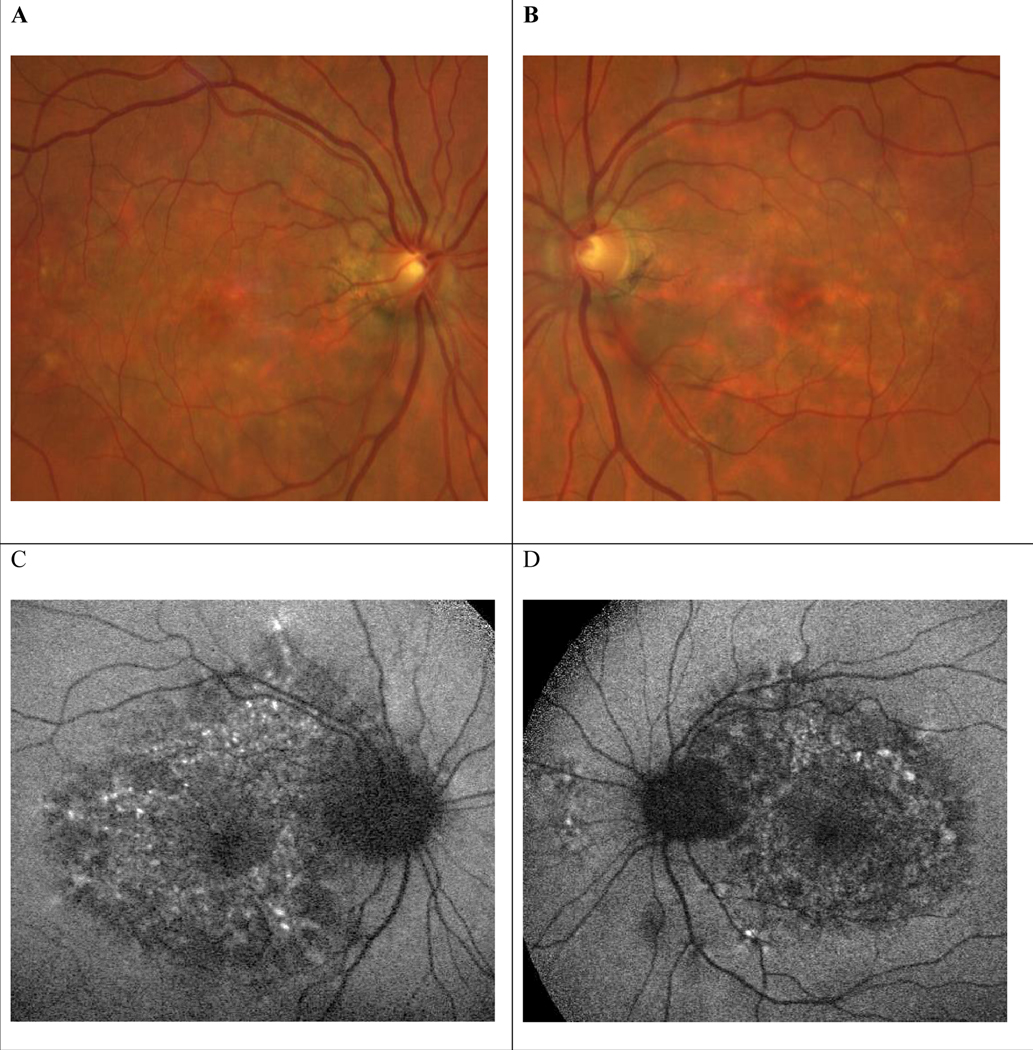
A and B, Color photographs from June 17, 2021, patient aged 55, showing yellowish deposits and macular pigment deposition. C and D, Fundus autofluorescence shows a densely packed array of macular hypo- and hyperautofluorescent spots involving the retina nasal to the optic disc OS > OD as well as a hypoautofluorescent peripapillary halo. OD indicates right eye; OS, left eye.
This case is illuminating for several reasons, most importantly for the way it shows the diagnostic and management dilemma faced by ophthalmologists in the face of this novel pigmentary maculopathy with its ability to mimic other retinal disorders. The classic teaching is that AMD is unlikely before age 50 and such a diagnosis should prompt further investigation. Late or exudative AMD is very uncommon before age 60.49 This patient was diagnosed with nAMD in the left eye almost a decade before the first report of PPS maculopathy and in the right eye more than 4 years before the first report of PPS maculopathy, and started a 9-year journey of receiving bilateral intravitreal injections in 2012, which she might need indefinitely. We believe the clinical scenario here is more common than thought and that similar cases will be uncovered over time. AMD is a much more established and common disease, so it is far more likely that such a presentation would be from AMD, especially in patients 55 and older. Additionally, PPS maculopathy may present after a patient no longer takes the drug, as in the case of this patient who had stopped taking pentosan the year before she was diagnosed with AMD at another institution. Therefore, if PPS was not in a patient’s medication list, the maculopathy might go unrealized unless the patient was specifically asked whether they took PPS in the past, and some might not recall this information. While knowing the etiology of CNV in nAMD or PPS maculopathy would not change management since both are treated with anti-VEGF therapy, this distinction may have implications for stopping PPS and may have medicolegal implications.
Regardless of the etiology of choroidal neovascular membranes (CNVM) in bilateral conditions (eg, AMD, PPS maculopathy, histoplasmosis), once a diagnosis of CNVM is made in 1 eye, a patient is more likely to have close monitoring of the fellow eye and receive anti-VEGF therapy in that eye early in the course of its development, as in the case of our patient. Starr and coworkers analyzed the Vestrum Database and found that 38% of these patients experienced conversion to nAMD in the fellow eye within the first 3 years of diagnosis.50
To our knowledge, this is only the third documented case of pentosan maculopathy complicated by CNV,33,44 and the first such case of bilateral, sequential neovascularization in both eyes of a single patient. Also, Huckfeldt and Vavvas described progression 6 years after discontinuation of PPS4 and this patient stopped PPS in 2010, developed CNV in the first eye in 2012 and continued to receive intravitreal anti-VEGF therapy 9 years later while maintaining 20/20 VA in each eye. We hope this case will alert clinicians to review their existing cases of AMD and conduct a careful medication and urological history to find cases of pentosan maculopathy which may have been misdiagnosed or which coexist with AMD.
5. Controversy and Directions for Future Research
We believe that chief among the open areas of study are development of workable screening guidelines, elucidation of why some patients exposed to pentosan get maculopathy and not others, and understanding how this disease may present in nonwhite populations.
5.1. Other Associations?
It now seems clear is that exposure to a high enough dose of PPS is a necessary but not sufficient condition in the development of this novel pigmentary maculopathy. The number of patients who have been exposed to sufficient doses of pentosan but do not have disease still outweighs those with observable or symptomatic maculopathy. Unless any use of the drug causes subclinical disease, it seems logical that these cases are only being discovered now, given the length of time required to develop maculopathy and the mildness of initial symptoms.
There may be a cofactor which predisposes some patients and not others to disease. We believe this is likely genetic in nature and further studies of common characteristics of patients who get pentosan maculopathy will help elucidate this. There are data that IC patients also carry diagnoses of irritable bowel syndrome, depression, or anxiety,5 and another study showed that 73% of patients reported pain at an area outside of the primary IC, indicating a possible centrally mediated mechanism for the pain.51 Warren and coworkers also found that 78% of patients with IC also had 2 or more of: fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, or Sicca syndrome when compared to matched controls. The authors concluded that either: 1) the patients shared a genetic or environmental risk factor and there was no direct relationship, 2) the associated syndromes were risk factors for IC, or 3) the associated syndrome and IC were different manifestations of the same pathophysiology.52 It seems possible that the subset of IC patients exposed to pentosan who developed maculopathy further share another genetic trait or traits.
Understanding the pathophysiology of PPS maculopathy will allow treatments to be developed for IC which may avoid that complication. Such an understanding may also shed light on key mechanisms causing AMD
5.2. Lack of Data on Other Ethnic/Racial Groups
What also bears mentioning is the paucity of data on pentosan maculopathy in people of color. In the studies to date that document race, 93% of patients are classified as white and 90% are female.27 To some degree this mirrors the characteristics of patients diagnosed with IC in the US,53 but the lack of data on other populations is troublesome for several reasons.
The overwhelming preponderance of white patients in these studies may generally be due to inequity in our health care system. It is well documented that racial and ethnic disparities exist on every level of health care,54 and this may be reflected in the difficulty patients of color have in being seen by a primary care doctor or urologist, being diagnosed with IC, being prescribed or able to afford Elmiron, and seeking the care of an ophthalmology subspecialist should they develop symptoms. While the systemic racism inherent in medicine may account for this difference, there may be another explanation as well.
The epidemiology and pathophysiology of IC is still poorly understood, and IC may present differently in different ethnic or racial groups. A retrospective review of the patient database at the Interstitial Cystitis Center at the New England Medical Center by Sant identified 17 minority women out of a total of 201 patients with a diagnosis of IC: 6 African American, 7 Hispanic, and 4 Asian.55 In this study, minority women responded to treatment in the same manner as their white counterparts but were symptomatic for a longer period before a diagnosis was made. A more recent review hints at the possibility that IC may present differently and have different natural histories in nonwhite populations.56
In their study from Japan, Ito and coworkers found that the diagnosis rate of IC in Japan was far lower than in Europe and North America.57 The authors suggested this difference might be due to racial differences in the disease, lifestyle factors, diagnostic patterns of urologists in the 2 countries, or lack of patient awareness of the disease.57 In his study from India, Mishra found that although IC was thought to be rare in India, the diagnosis rate was increasing and that Indian patients present with slightly different symptoms: a large percentage had obstructive symptoms, unusual urinary symptoms, and anal discomfort.58
Zhang and coworkers sought to quantify some of the differences between white, African American, and native Chinese women. They measured urine levels of 3 biomarkers previously established in whites: antiproliferative factor (APF), heparin-binding epidermal growth factor–like growth factor (HB-EGF), and epidermal growth factor (EGF).59 They found that women from these 3 racial groups had similar levels of all 3.
The implications in clinical ophthalmology for PPS maculopathy parallel the situation with other disorders. Hydroxychloroquine macular toxicity usually presents in a parafoveal distribution in Europeans, African Americans, and Hispanics, but patients of Asian descent more likely shows damage in a more peripheral extramacular distribution near the arcades. These differences were codified in the 2016 American Academy of Ophthalmology Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy.60 AMD provides a similar correlate: polypoidal choroidal vasculopathy, a subclassification of type 1 CNV with a large aneurysmal component, is observed more commonly in African and Asian people, with a reported frequency of 22% to 62% among people with AMD in Asian populations.61
It remains to be seen whether PPS maculopathy will present differently in Asians or other nonwhite groups, but this possibility is something clinicians need to be cognizant of. In the meantime, more data needs to be gathered from these populations.
6. Conclusions
Pentosan maculopathy is a sight-threatening disease and can cause great impact on quality of life in severe cases. The same can be said for interstitial cystitis. This is the dilemma with which clinicians are now faced.
“Elmiron is my magical drug that allowed me to get my life back.”
“This drug ruined my life.”
These statements, made by 2 different patients at our institution, highlight the difficulty clinicians have when deciding whether to advise patients to discontinue PPS if they develop retinal or visual problems. Do doctors advise patients to stop a potentially life-altering drug if they have even mild visual symptoms? How can they be certain a patient has PPS maculopathy and not another disease, or both?
Our review of the literature confirms the general consensus around this disease: it is caused by higher cumulative doses of pentosan and presents in a recognizable manner on multimodal imaging along with symptoms consistent with rod dysfunction, similar to AMD.62,63 We also highlight the fact that pentosan maculopathy is a great masquerade syndrome for inherited retinal dystrophies and AMD, and this can have a negative impact on patients with an incorrect diagnosis. It is conceivable that patients on pentosan are being followed year after year for AMD, are experiencing progressive RPE atrophy, and are going unnoticed.
What is lacking thus far is a comprehensive search for commonalities between patients afflicted by pentosan maculopathy. The development of an effective screening protocol based on analysis of shared patient characteristics is a valuable area for future study. Ophthalmologists should have a high index of suspicion for this vision-threatening condition in all their new patients taking pentosan, but we believe it is more important that they scrutinize their existing patient base, especially those with AMD, for potential misdiagnoses.
Acknowledgments
Funding/Support: This work was supported by NEI R01 EY026547, P30 EY025580
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Pearce WA, Chen R, Jain N. Pigmentary maculopathy associated with chronic exposure to pentosan polysulfate sodium. Ophthalmology. 2018;125:1793–802. doi: 10.1016/j.ophtha.2018.04.026 [DOI] [PubMed] [Google Scholar]
- 2.Ricca AM, Han IC, Sohn EH. Stargardt disease masquerades. Curr Opin Ophthalmol. 2021;32:214–224. doi: 10.1097/ICU.0000000000000750 [DOI] [PubMed] [Google Scholar]
- 3.Sohn EH, Mullins RF, Stone EM. Ryan’s Retina. 6th ed. New York, US: Elsevier; 2018;44:953–996. [Google Scholar]
- 4.Huckfeldt RM, Vavvas DG. Progressive maculopathy after discontinuation of pentosan polysulfate sodium. Ophthalmic Surg Lasers Imaging Retina. 2019;50:656–659. doi: 10.3928/23258160-20191009-10 [DOI] [PubMed] [Google Scholar]
- 5.Hanno PM, Erickson D, Moldwin R, et al. American Urological Association. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193:1545–1553. doi: 10.1016/j.juro.2015.01.086 [DOI] [PubMed] [Google Scholar]
- 6.Hanif AM, Yan J, Jain N. Pattern dystrophy: an imprecise diagnosis in the age of precision medicine. Int Ophthalmol Clin. 2019;59:173–194. doi: 10.1097/IIO.0000000000000262 [DOI] [PubMed] [Google Scholar]
- 7.Christiansen JS, Barnes AC, Berry DE, et al. Pentosan polysulfate maculopathy versus age-related macular degeneration: comparative assessment with multimodal imaging. Can J Ophthalmol. 2021. Online ahead of print. doi: 10.1016/j.jcjo.2021.02.007 [DOI] [PubMed] [Google Scholar]
- 8.Rothrock NE, Lutgendorf SK, Hoffman A, et al. Depressive symptoms and quality of life in patients with interstitial cystitis. J Urol. 2002;167:1763–1767. [PubMed] [Google Scholar]
- 9.Rothrock N, Lutgendorf SK, Kreder KJ. Coping strategies in patients with interstitial cystitis: relationships with quality of life and depression. J Urol. 2003;169:233–236. doi: 10.1097/01.ju.0000037669.20893.f7 [DOI] [PubMed] [Google Scholar]
- 10.Kusek JW, Nyberg LM. The epidemiology of interstitial cystitis: is it time to expand our definition? Urology. 2001;57(suppl 6A):95–99. [DOI] [PubMed] [Google Scholar]
- 11.Patnaik SS, Laganà AS, Vitale SG, et al. Etiology, pathophysiology and biomarkers of interstitial cystitis/painful bladder syndrome. Arch Gynecol Obstet. 2017;295:1341–1359. doi: 10.1007/s00404-017-4364-2 [DOI] [PubMed] [Google Scholar]
- 12.Marcu I, Campian EC, Tu FF. Interstitial cystitis/bladder pain syndrome. Semin Reprod Med. 2018;36:123–135. doi: 10.1055/s-0038-1676089 [DOI] [PubMed] [Google Scholar]
- 13.Cui X, Jing X, Lutgendorf SK, et al. Cystitis-induced bladder pain is toll-like receptor 4 dependent in a transgenic autoimmune cystitis murine model: a MAPP Research Network animal study. Am J Physiol Renal Physiol. 2019;317:F90–F98. doi: 10.1152/ajprenal.00017.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster HE Jr, Hanno PM, Nickel JC, et al. Interstitial Cystitis Collaborative Research Network. Effect of amitriptyline on symptoms in treatment naïve patients with interstitial cystitis/painful bladder syndrome. J Urol. 2010;183:1853–1858. doi: 10.1016/j.juro.2009.12.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giusto LL, Zahner PM, Shoskes DA. An evaluation of the pharmacotherapy for interstitial cystitis. Expert Opin Pharmacother. 2018;19:1097–1108. doi: 10.1080/14656566.2018.1491968 [DOI] [PubMed] [Google Scholar]
- 16.Parsons CL, Schmidt JD, Pollen JJ. Successful treatment of interstitial cystitis with sodium pentosanpolysulfate. J Urol. 1983;130:51–53. doi: 10.1016/s0022-5347(17)50948-9 [DOI] [PubMed] [Google Scholar]
- 17.Malde S, Palmisani S, Al-Kaisy A, et al. Guideline of guidelines: bladder pain syndrome. BJU Int. 2018;122:729–743. doi: 10.1111/bju.14399 [DOI] [PubMed] [Google Scholar]
- 18.Nickel JC, Moldwin R. FDA BRUDAC 2018 Criteria for interstitial cystitis/bladder pain syndrome clinical trials: future direction for research. J Urol. 2018;200:39–42. doi: 10.1016/j.juro.2018.02.011 [DOI] [PubMed] [Google Scholar]
- 19.Anderson VR, Perry CM. Pentosan polysulfate: a review of its use in the relief of bladder pain or discomfort in interstitial cystitis. Drugs. 2006;66:821–835. doi: 10.2165/00003495-200666060-00006 [DOI] [PubMed] [Google Scholar]
- 20.Nickel JC, Herschorn S, Whitmore KE, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: insights from a randomized, double-blind, placebo-controlled study. J Urol. 2015;193:857–862. doi: 10.1016/j.juro.2014.09.036 [DOI] [PubMed] [Google Scholar]
- 21.Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810–815. doi: 10.1097/01.ju.0000083020.06212.3d [DOI] [PubMed] [Google Scholar]
- 22.United States Food and Drug Administration. Elmiron®−100 Mg (Pentosan Polysulfate Sodium) Capsules Prescribing Information. Available from https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020193s014lbl.pdf. Accessed September 26, 2021.
- 23.Hanif AM, Shah R, Yan J, et al. Strength of association between pentosan polysulfate and a novel maculopathy. Ophthalmology. 2019;126:1464–1466. doi: 10.1016/j.ophtha.2019.04.024 [DOI] [PubMed] [Google Scholar]
- 24.Vora RA, Patel AP, Melles R. Prevalence of maculopathy associated with long-term pentosan polysulfate therapy. Ophthalmology. 2020; 127:835–836. doi: 10.1016/j.ophtha.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Au A, Gunnemann F, et al. Pentosan-associated maculopathy: prevalence, screening guidelines, and spectrum of findings based on prospective multimodal analysis. Can J Ophthalmol. 2020; 55:116–125. doi: 10.1016/j.jcjo.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 26.Higgins K, Welch RJ, Bacorn C, et al. Identification of patients with pentosan polysulfate sodium-associated maculopathy through screening of the electronic medical record at an academic center. J Ophthalmol. 2020;2020:8866961. doi: 10.1155/2020/8866961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindeke-Myers A, Hanif AM, Nieraj Jain. Pentosan polysulfate maculopathy. Survey of Ophthalmology. 2022;67:83–96. doi: 10.1016/j.survophthal.2021.05.005 [DOI] [PubMed] [Google Scholar]
- 28.Barnett JM, Jain N. Potential new onset clinically detectable pentosan polysulfate maculopathy years after drug cessation. Retin Cases Brief Rep. 2021. Online ahead of print. doi: 10.1097/ICB.0000000000001090 [DOI] [PubMed] [Google Scholar]
- 29.Hadad A, Helmy O, Leeman S, et al. A novel multimethod image analysis to quantify pentosan polysulfate sodium retinal toxicity. Ophthalmology. 2020;127:429–431. doi: 10.1016/j.ophtha.2019.10.013 [DOI] [PubMed] [Google Scholar]
- 30.Uner OE, Shah MK, Jain N. Pentosan polysulfate and vision: findings from an international survey of exposed individuals. RETINA. 2021. Publish ahead of print. doi: 10.1097/IAE.0000000000003078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyons RJ, Brower J, Jain N. Visual Function in Pentosan Polysulfate Sodium Maculopathy. Invest Ophthalmol Vis Sci. 2020;61:33. doi: 10.1167/iovs.61.13.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyons R, Hanif A, Jain N. Pentosan polysulfate maculopathy: comprehensive functional analysis and structure-function correlation. Invest Ophthalmol Vis Sci. 2020;61:1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanif AM, Armenti ST, Taylor SC, et al. Phenotypic spectrum of pentosan polysulfate sodium-associated maculopathy: a multicenter study. JAMA Ophthalmol. 2019;137:1275–1282. doi: 10.1001/jamaophthalmol.2019.3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuchman EH, Ge Y, Lai A, et al. Pentosan polysulfate: a novel therapy for the mucopolysaccharidoses. PLoS One. 2013;8:e54459. doi: 10.1371/journal.pone.0054459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaffney PJ, Marsh NA. The effect of pentosan polysulphate (SP54) on the human fibrinolytic system. Folia Haematol Int Mag Klin Morphol Blutforsch. 1986;113:262–271. [PubMed] [Google Scholar]
- 36.Greenlee T, Hom G, Conti T, et al. Re: Pearce et al.: Pigmentary maculopathy associated with chronic exposure to pentosan polysulfate sodium (Ophthalmology. 2018;125:1793–1802). Ophthalmology. 2019;126:e51. doi: 10.1016/j.ophtha.2018.12.037 [DOI] [PubMed] [Google Scholar]
- 37.Hochmann S, Kaslin J, Hans S, et al. Fgf signaling is required for photoreceptor maintenance in the adult zebrafish retina. PLoS One. 2012;7:e30365. doi: 10.1371/journal.pone.0030365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yusuf IH, Charbel Issa P, Lotery AJ. Pentosan polysulfate maculopathy-prescribers should be aware. JAMA Ophthalmol. 2020;138:900–902. doi: 10.1001/jamaophthalmol.2020.2364 [DOI] [PubMed] [Google Scholar]
- 39.Pouw AE, Greiner MA, Coussa RG, et al. Cell-matrix interactions in the eye: from cornea to choroid. Cells. 2021;10:687. doi: 10.3390/cells10030687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abou-Jaoude M, Davis A, Fraser C, et al. New insights into pentosan polysulfate maculopathy. Ophthalmic Surg Lasers Imaging Retina. 2021;52:13–22. [DOI] [PubMed] [Google Scholar]
- 41.Sohn EH, Flamme-Wiese MJ, Whitmore SS, et al. Choriocapillaris degeneration in geographic atrophy. Am J Pathol. 2019;189:1473–1480. doi: 10.1016/j.ajpath.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitmore SS, Sohn EH, Chirco KR, et al. Complement activation and choriocapillaris loss in early AMD: implications for pathophysiology and therapy. Prog Retin Eye Res. 2015;45:1–29. doi: 10.1016/j.preteyeres.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Larochellière E, Bourgault S. Pentosan polysulfate sodium-induced pigmentary maculopathy with non-leaking cystoid macular edema successfully treated with anti-VEGF therapy. Retin Cases Brief Rep. 2020. Online ahead of print. doi: 10.1097/ICB.0000000000001013. [DOI] [PubMed] [Google Scholar]
- 44.Mishra K, Patel TP, Singh MS. Choroidal neovascularization associated with pentosan polysulfate toxicity. Ophthalmol Retina. 2020;4:111–113. doi: 10.1016/j.oret.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 45.Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389:2338–2348. doi: 10.1016/S0140-6736(17)31491-5 [DOI] [PubMed] [Google Scholar]
- 46.Jain N, Li AL, Yu Y, et al. Association of macular disease with long-term use of pentosan polysulfate sodium: findings from a US cohort. Br J Ophthalmol. 2019;104:1093–1097. doi: 10.1136/bjophthalmol-2019-314765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnes AC, Hanif AM, Jain N. Pentosan polysulfate maculopathy versus inherited macular dystrophies: comparative assessment with multimodal imaging. Ophthalmol Retina. 2020;4:1196–1201. doi: 10.1016/j.oret.2020.05.008 [DOI] [PubMed] [Google Scholar]
- 48.Cleland SC, Domalpally A, Liu Z, et al. Second Carotenoids in Age-Related Eye Disease Study Investigators. Reticular Pseudodrusen Characteristics and Associations in the Carotenoids in Age-Related Eye Disease Study 2 (CAREDS2), an Ancillary Study of the Women’s Health Initiative. Ophthalmol Retina. 2021;5:721–729. doi: 10.1016/j.oret.2020.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell P, Liew G, Gopinath B, et al. Age-related macular degeneration. Lancet. 2018;392:1147–1159. doi: 10.1016/S0140-6736(18)31550-2 [DOI] [PubMed] [Google Scholar]
- 50.Starr MR, Xu D, Boucher N, et al. Characterizing progression to neovascular amd in fellow eyes of patients treated with intravitreal anti-VEGF injections. Ophthalmic Surg Lasers Imaging Retina. 2021;52:123–128. doi: 10.3928/23258160-20210302-02 [DOI] [PubMed] [Google Scholar]
- 51.Tripp DA, Nickel JC, Wong J, et al. Mapping of pain phenotypes in female patients with bladder pain syndrome/interstitial cystitis and controls. Eur Urol. 2012;62:1188–1194. [DOI] [PubMed] [Google Scholar]
- 52.Warren JW, Howard FM, Cross RK, et al. Antecedent nonbladder syndromes in case-control study of interstitial cystitis/painful bladder syndrome. Urology. 2009;73:52–57. [DOI] [PubMed] [Google Scholar]
- 53.Teichman JM, Parsons CL. Contemporary clinical presentation of interstitial cystitis. Urology. 2007;69(4 Suppl):41–47. doi: 10.1016/j.urology.2006.08.1111 [DOI] [PubMed] [Google Scholar]
- 54.Wheeler SM, Bryant AS. Racial and ethnic disparities in health and health care. Obstet Gynecol Clin North Am. 2017;44:1–11. doi: 10.1016/j.ogc.2016.10.001 [DOI] [PubMed] [Google Scholar]
- 55.Sant GR. Interstitial cystitis in minority women. J Assoc Acad Minor Phys. 1993;4:89–92. [PubMed] [Google Scholar]
- 56.Dallas KB, Bresee C, De Hoedt A, et al. Demographic differences and disparities in the misdiagnosis of interstitial cystitis/bladder pain syndrome in a national cohort of VA patients. Urology. 2021:S0090–4295(21)00709–3. doi: 10.1016/j.urology.2021.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ito T, Miki M, Yamada T. Interstitial cystitis in Japan. BJU Int 2000;86:634–637. [DOI] [PubMed] [Google Scholar]
- 58.Mishra NN. Clinical presentation and treatment of bladder pain syndrome/interstitial cystitis (BPS/IC) in India. Transl Androl Urol. 2015;4:512–523. doi: 10.3978/j.issn.2223-4683.2015.10.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang CO, Li ZL, Shoenfelt JL, et al. Comparison of APF activity and epithelial growth factor levels in urine from Chinese, African-American, and white American patients with interstitial cystitis. Urology. 2003;61:897–901. doi: 10.1016/s0090-4295(02)02597-9 [DOI] [PubMed] [Google Scholar]
- 60.Marmor MF, Kellner U, Lai TY, et al. American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118:415–422. doi: 10.1016/j.ophtha.2010.11.017 [DOI] [PubMed] [Google Scholar]
- 61.Wong CW, Yanagi Y, Lee WK, et al. Age-related macular degeneration and polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res. 2016;53:107–139. [DOI] [PubMed] [Google Scholar]
- 62.Owsley C, Jackson GR, Cideciyan AV, et al. Psychophysical evidence for rod vulnerability in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:267–273. [PubMed] [Google Scholar]
- 63.Mullins RF, McGwin G Jr, Searcey K, et al. The ARMS2 A69S polymorphism is associated with delayed rod-mediated dark adaptation in eyes at risk for incident age-related macular degeneration. Ophthalmology. 2019;126:591–600. doi: 10.1016/j.ophtha.2018.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]