Abstract
Closed-loop deep brain stimulation is a novel form of therapy that has shown benefit in preliminary studies and may be clinically available in the near future. Initial closed-loop studies have primarily focused on responding to sensed biomarkers with adjustments to stimulation amplitude, which is often perceptible to study participants depending on the slew or “ramp” rate of the amplitude changes. These subjective responses to stimulation ramping can result in transient side effects, illustrating that ramp rate is a unique safety parameter for closed-loop neural systems. This presents a challenge to the future of closed-loop neuromodulation systems: depending on the goal of the control policy, clinicians will need to balance ramp rates to avoid side effects and keep the stimulation therapeutic by responding in time to affect neural dynamics. In this paper, we demonstrate the results of an initial investigation into methodology for finding safe and tolerable ramp rates in four people with Parkinson’s disease (PD). Results suggest that optimal ramp rates were found more accurately during varying stimulation when compared to simply toggling between maximal and minimal intensity levels. Additionally, switching frequency instantaneously was tolerable at therapeutic levels of stimulation. Future work should focus on including optimization techniques to find ramp rates.
I. Introduction
Open-loop deep brain stimulation (DBS) is an effective treatment for symptoms of Parkinson’s disease (PD) that requires optimization by a clinician. Typically, the therapeutic window (i.e., the range of intensities that provide clinical benefit but do not cause side effects) is determined for each stimulation contact using a trial-and-error process of titrating stimulation intensity (i.e., amplitude per pulse) [1], [2]. This process enables the clinician to maximize the stimulation settings for clinical efficacy while minimizing side effects.
Recent advances in technology (i.e., sensing neurostimulators) have enabled the investigation of closed-loop DBS for PD. The algorithms have mostly focused on adapting stimulation intensity in response to a biomarker (e.g., beta band oscillations) [3]–[5], while adapting other parameters (e.g., stimulation frequency) may become more common in future closed-loop paradigms [6], [7]. However, these algorithms will expand the number of parameters that need to be tuned if they become clinically available, thus increasing the complexity of finding the therapeutic window [8], [9].
An important parameter of these algorithms is the slew or “ramp” rates that can be perceptible to patients in the form of transient side effects (e.g., paresthesias), which can be unpleasant or even intolerable. This means that ramp rates are a unique safety parameter for closed-loop DBS that should be considered carefully. Furthermore, the control policies for these algorithms vary vastly, from millisecond precision to adapting over the course of hours [3]–[6]. Thus, adequate adjustment of these ramp rates will be necessary to not only balance side effects and therapeutic benefit, but also respond to the biomarkers of interest.
Currently, a standardized process for determining the ramp rates for a closed-loop algorithm has not been established. In this paper we provide: (1) an application to enable clinicians and researchers to search for effective ramp rates and test optimization methods and (2) initial results from multiple approaches for optimizing ramp rates in a small cohort of people with PD.
II. Methods
A. Ramp Rate Testing Application
A custom C# application was developed to facilitate research staff explorations of ramp rate selection with study participant input and feedback. The application was built to control the implanted investigational-use Summit™ RC+S sensing neurostimulator (Medtronic PLC) using the Medtronic-provided C# API (Fig. 1). The RC+S has an adaptive group with four stimulation “programs” (each comprised of a cathode/anode pair with a unique stimulation pulse configuration) that can be utilized simultaneously and independently for closed-loop algorithms. Each program has its own rise and fall ramp values (in mA/sec) that can be adjusted within limits set on the clinician programmer. The application user interface (UI, Fig. 2) allows the research staff to utilize the on-board state table to set up adaptation between two stimulation states for testing with the study participant. These states represent “on-board” target stimulation configurations that the RC+S can utilize on command [10]. In the case of states with different amplitudes, the current is incrementally ramped at the predefined rate toward the target by the RC+S firmware. In the case of two states with different frequencies, the frequency is instantaneously switched to the new state’s target. By instructing the RC+S to toggle between these target stimulation states, the application allows for researchers to provide stimulation changes to the patient, either ramping of amplitude or rapid frequency changes, to explore the tolerability of different stimulation paradigms.
Figure 1:
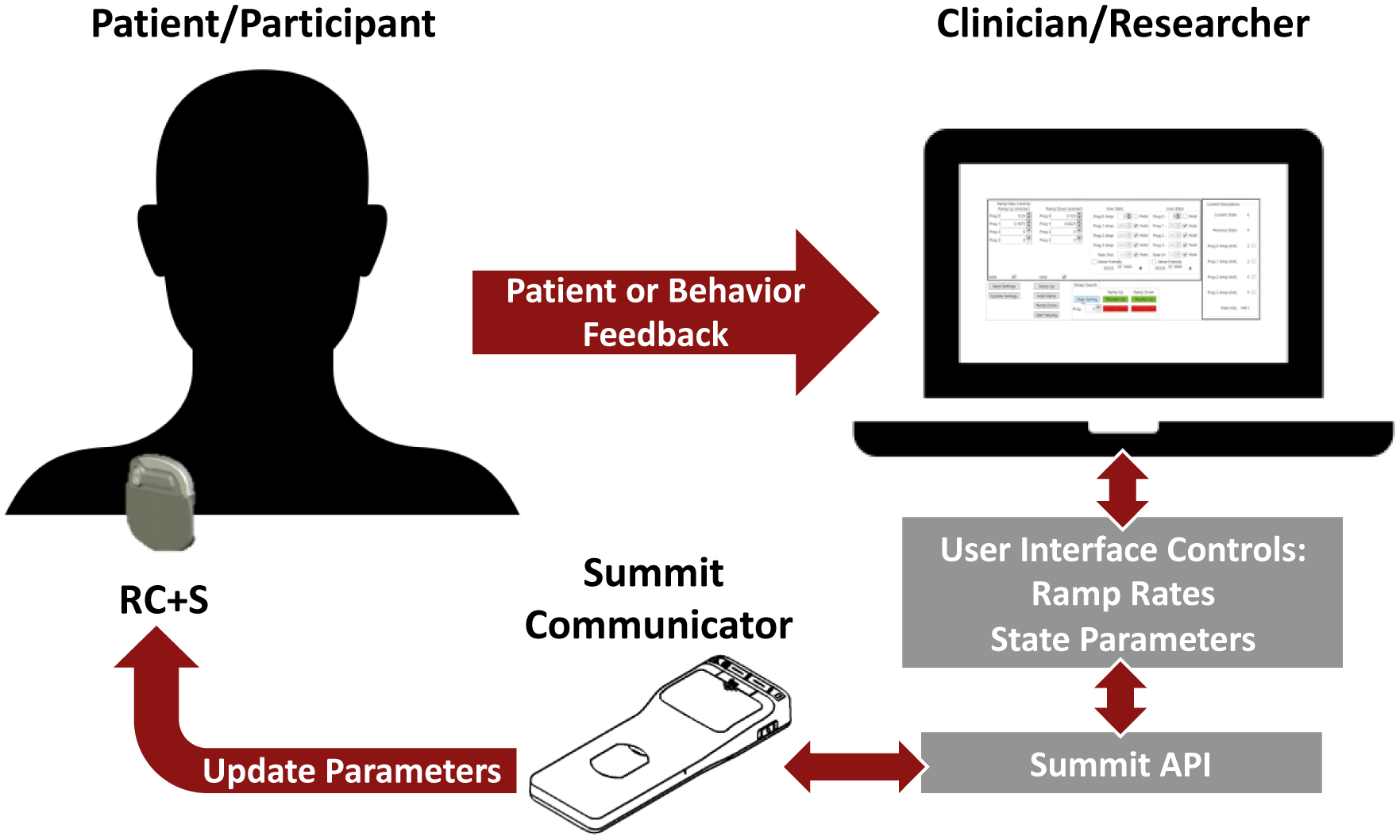
Block diagram of the ramp rate process using the Medtronic Summit™ RC+S. First the clinician or researcher use a C# application to communicate parameters through the Summit™ API to the Summit™ RC+S. Once the ramp rate has been tested, the patient or participant provide feedback to the doctor or researcher, and the process continues until safe and tolerable ramp rates are found.
Figure 2:
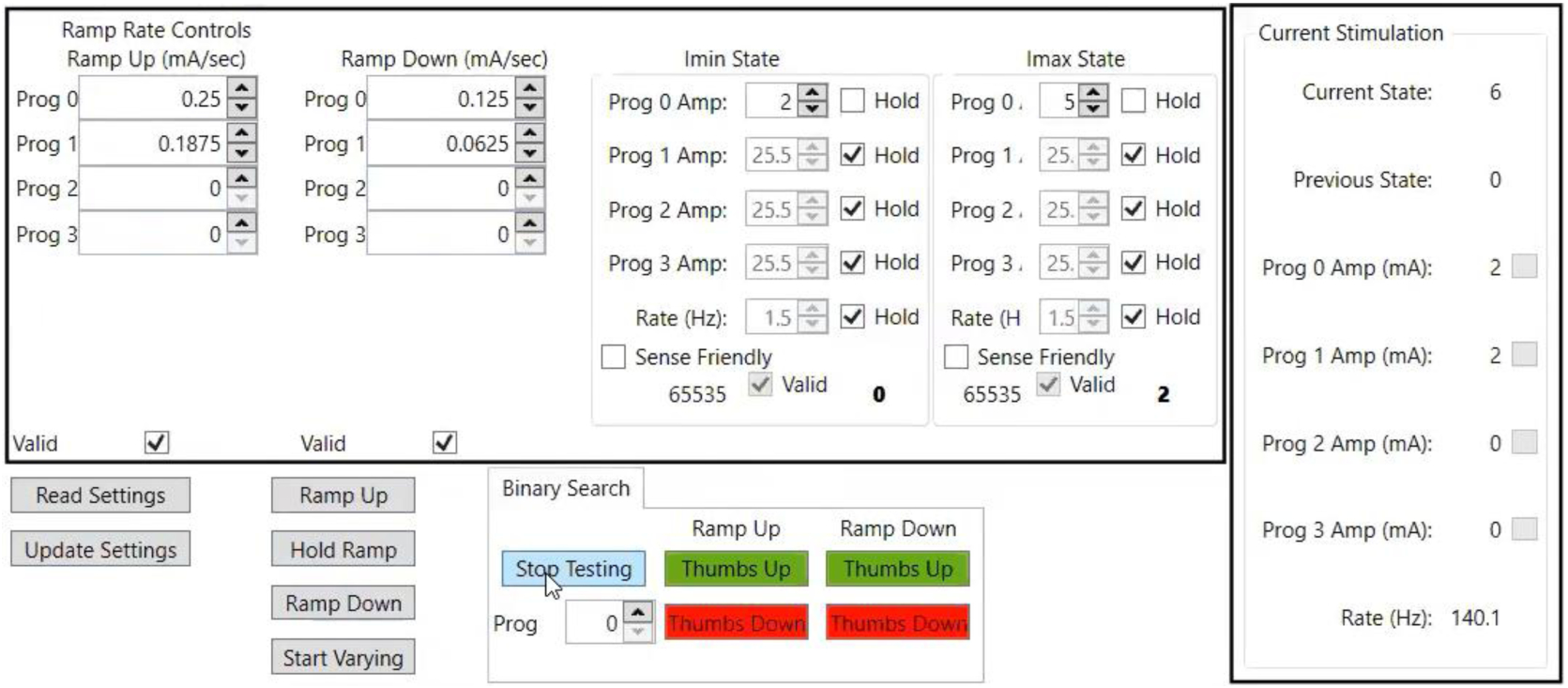
The ramp rate UI. The user is given access to the ramp rates for each (in mA/sec). Two states (currently labeled and Imin and Imax) can be set up to either vary stimulation intensity or frequency, and multiple programs can be adjusted simultaneously. The user should start by clicking “Read Settings”, which will show the current settings configured on the device. Once the necessary adjustments are made, the user can then “Update Settings”, and if valid the UI will give the user the ability to manually “Ramp Up,” Hold Ramp, or “Ramp Down” between the configured states. There is also the option to use the “Start Varying” button, which will intermittently switch between the two states at varying intervals, which may be more indicative of what would happen during closed-loop DBS. Ramp rates can be adjusted manually, or a binary search with feedback buttons (for ramp up or down) will give the next value to test. Finally, the real time read out of the current state, program amplitudes, and stimulation frequency are displayed on the right.
Once the user has selected initial settings for ramp rates and the stimulation states are updated on the device, the user is able to test ramps in two ways. First, they can toggle a manual control of ramps between defined maximum/minimum stimulation amplitudes to evaluate the maximal rate of ramp given the defined settings. Alternatively, the application can deliver ramping stimulation at intermittent timing intervals designed to more closely match the dynamic behavior of ramping delivered by a closed-loop paradigm (“intermittent varying”, Fig. 3). Timing intervals of 3–10 seconds between state changes were used for the protocols in this study. For safety reasons, in the case where a patient has a negative/adverse response to a chosen ramp, the application also provides the study staff a “Hold Ramp” button to act as an immediate cessation of ramping activity. The application provides the user with the ability to determine the next tested ramp parameters either in a trial-and-error process or utilize a simple binary search. The binary search uses positive and negative feedback buttons (“Thumbs Up” or “Thumbs Down” respectively) to narrow the search until an adequate ramp rate for each patient is found. The interface provides this guidance functionality in a tab structure to allow for future extensions.
Figure 3:
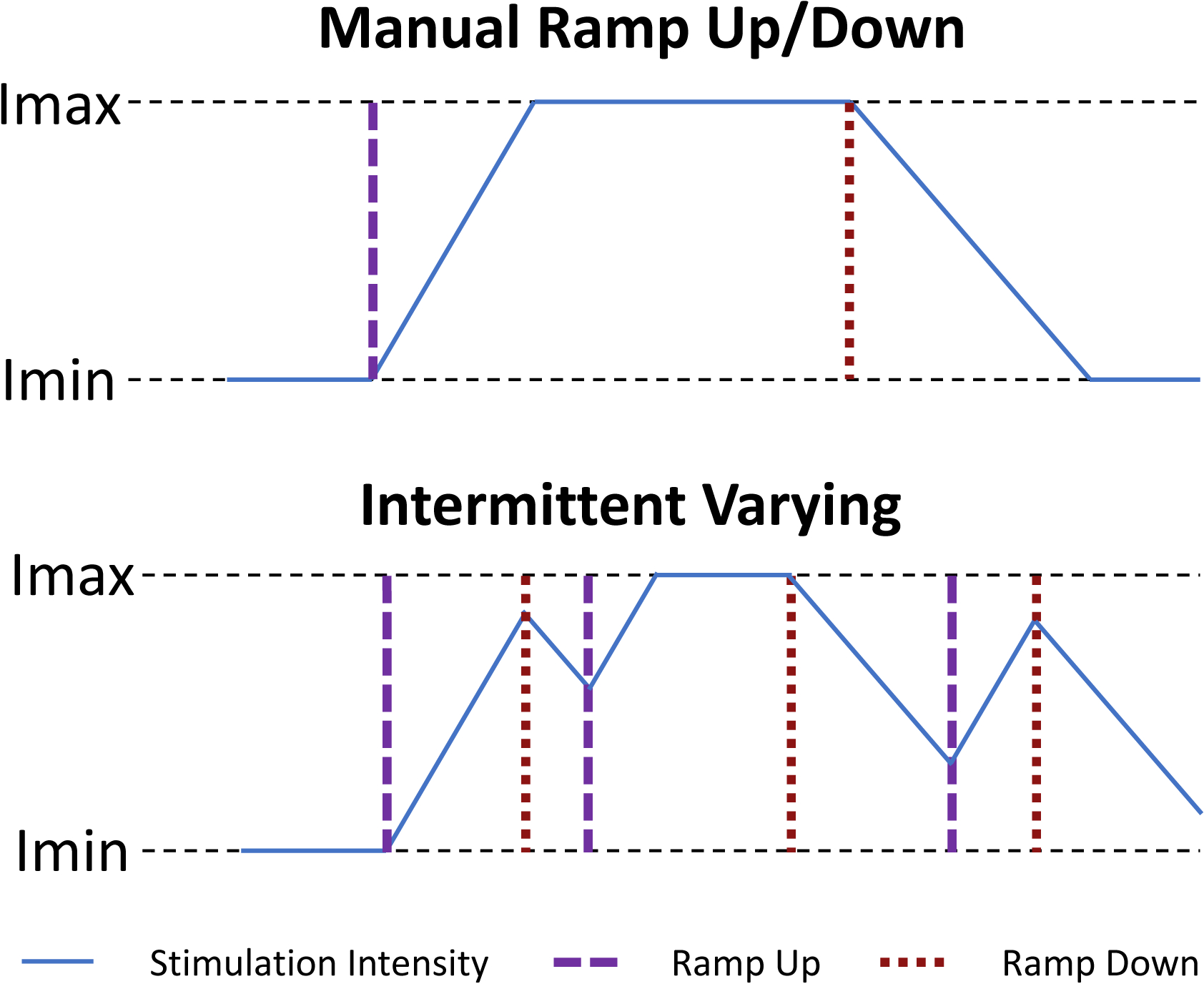
Example of stimulation intensity adapting in the manual vs. intermittent ramping paradigms. Imax is the maximum stimulation intensity that has therapeutic benefit but no side effects. Imin is the minimally therapeutic stimulation intensity.
B. Participants
Ramp rates and switching frequencies were evaluated in 4 males with PD (age 63.3 ± 3.5 years, disease duration 10.8 ± 2.1 years). All participants were implanted with the Medtronic Summit™ RC+S system (Medtronic PLC) with bilateral 3389 leads placed in the subthalamic nucleus (STN). All participants signed informed written consent and all protocols were FDA IDE and Stanford University Institutional Review Board approved.
C. Testing Procedure
In Participant 1, the binary search was used for one STN and a trial-and-error process was used for the other STN. A binary search was used to find ramps that were safe and tolerable for Participants 2–4. Convergence was satisfied when the fastest, safe, and tolerable ramp rate was found. Manually ramping between the Imin and Imax states was used for Participants 1 and 2 (Imin = minimally therapeutic stimulation intensity, Imax = maximum stimulation intensity that is therapeutic without side effects), while the “Intermittent Varying” control was used for Participants 3 and 4. The initial starting values for ramp rates were 0.25 mA/sec up and 0.125 mA/sec down for Participants 1–3. These reflect the same ramp rates that were found to be safe and tolerable using the Medtronic Activa™ PC+S (0.1V/0.4s up and 0.1V/0.8s down, [3], [11]), but they were converted to mA assuming a 1kΩ electrode impedance. Impedances values from the day of testing were used for this conversion for Participant 4. All ramp rates for adapting intensity were tested at a stimulation frequency of 140 Hz.
Switching stimulation frequency from 140 Hz to 60 Hz (and vice versa) via an instantaneous toggle was tested in a trial-and-error process while slowly increasing stimulation in 0.5 mA increments within both STNs simultaneously until both stimulation intensities were at Imax or side effects were observed. These two frequencies were used as 60 Hz might be better than 140 Hz for freezing of gait symptoms in PD, which will be tested in a closed-loop DBS algorithm [6], [7], [12], [13]. Note, a trial-and-error approach of slowly increasing stimulation intensity was used in this case because stimulation frequency switches instantaneously on the RC+S and there were no prior values from previous studies to use as a starting point. If a paresthesia was observed in both sides of the body simultaneously, then the prior setting that was tested was considered to be safe and tolerable. All tests were performed with the participant seated at rest.
III. Results
Safe and tolerable ramp rates were found for all participants (Table 1). Additionally, frequency switching was tolerable at therapeutic levels of stimulation for all participants. Typically, side effects were only observed while the system was ramping up stimulation intensity or when it was increasing stimulation frequency from 60 Hz to 140 Hz. In general, the binary search method worked well for all participants. In Participant 1, a trial-and-error process was used for the right STN and took five iterations to find a safe and tolerable ramp rate. In comparison, a binary search approach was able to find a safe and tolerable ramp rate within two iterations on the left STN, although two additional tests were performed to find a ramp that was still tolerable but close to the starting value of 0.25 mA/sec. Using impedance values from the day of the experiment for ramp rate conversion rather than assuming a 1kΩ electrode impedance resulted in only one iteration being needed for Participant 4. The manual ramping that was used for the first two participants resulted in more iterations for Participant 2 because paresthesias were observed when the ramp got close to or was at Imax. This occurred even when Imax was reduced by 0.2 mA increments from 3.4 mA to 2.8 mA. For the two participants where intermittent varying control was used, paresthesias were observed during the first set of changes in stimulation intensity, but this effect went away as the controller continued to adapt. Frequency switching was performed at Imax in 1 STN, while other STNs were all within intensities that were near therapeutic. All but one STN reached intensities that were equal to or greater than clinical settings.
Table 1:
Results of the ramp rate and frequency switch testing. “T&E” = trial-and-error, “Binary” = binary search for the search types.
| Participant No. | STN | Search Type | Ramp Type | Iterations to Find Ramp Rate | Final Imin (mA) | Final Imax (mA) | Ramp Rate Up (mA/sec) | Ramp Rate Down (mA/sec) | Clinical Stimulation Amplitude (mA) | Max Tolerable Intensity for frequency Switch (mA) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LSTN | Binary | Manual | 4 | 0.8 | 3 | 0.2188 | 0.2150 | 1.8 | 2.8 |
| 1 | RSTN | T&E | Manual | 5 | 0.9 | 3.8 | 0.0625 | 0.1250 | 2.2 | 2.8 |
| 2 | LSTN | Binary | Manual | 9 | 0.9 | 2.8 | 0.2500 | 0.1250 | 2.4 | 2.8 |
| 2 | RSTN | Binary | Manual | 6 | 1.1 | 3.8 | 0.0950 | 0.1250 | 3.2 | 3.5 |
| 3 | LSTN | Binary | Varying | 3 | 1.8 | 3.6 | 0.0625 | 0.1250 | 2.2 | 3.0 |
| 3 | RSTN | Binary | Varying | 2 | 2.0 | 3.9 | 0.1250 | 0.1250 | 2.9 | 1.8 |
| 4 | LSTN | Binary | Varying | 1 | 2.0 | 4.0 | 0.1570 | 0.0785 | 2.4 | 3.5 |
| 4 | RSTN | Binary | Varying | 1 | 2.1 | 4.2 | 0.1420 | 0.0711 | 2.6 | 3.6 |
IV. Discussion
In this paper, we presented a method for testing ramp rates for deep brain stimulation and provided preliminary results from people with PD. Safe and tolerable ramp rates of stimulation intensity were found for all participants. Side effects were typically observed while increasing stimulation intensity/frequency due to the increase in stimulation energy. Using a binary search and evaluating ramp rates during varying states, as opposed to a single go to state command, was more effective in finding ramp rates. Narrowing the initial starting values of the binary search can also reduce search time, including using electrode impedances to convert previously established ramp rates. Lastly, switching stimulation frequency was possible in all participants suggesting that this paradigm is possible for future closed-loop algorithms.
These data revealed some important principles of testing ramp rates. Namely, it is clear that performing manual ramps from a minimum stimulation intensity to a maximum stimulation intensity may not be indicative of what is safe and tolerable when the system is adapting as it would during closed-loop stimulation. In this study, we used a predetermined set of state changes, but there could also be an option for adapting based on a biomarker (e.g., beta oscillations) in real time. The advantage of using predetermined states is the ability to more directly test specific cases (e.g., adapting in longer and shorter intervals) more efficiently while also mimicking the dynamic state changes that occur during real-time closed loop DBS.
Similar to other stimulation parameters, it will be important to find ramp rates that not only balance side effects but also keep stimulation therapeutic. Aside from some of the initial papers[3]–[5], this is a parameter space that has not been sufficiently explored. The methods presented in this work provide the initial framework for investigating the configuration of ramp rates, and could also be used to test ramp rates on multiple time scales. It is feasible that in addition to patient feedback, behavioral metrics (e.g., axial movements or speech) could also be included in the search to ensure that both pieces of information are taken into account. Moreover, a binary search is the most basic form of an optimal search, but this tool can be expanded to utilize optimization techniques that could minimize a multivariable cost function.
The ability to do instantaneous frequency switching is promising for future algorithm design. There is evidence that certain frequencies may be better for some symptoms but not others. For example, there is evidence that 60 Hz stimulation may be more beneficial for freezing of gait symptoms, but not as effective for tremor. Thus, a closed-loop system that switches to 60 Hz only during freezing episodes would be beneficial. The important result here is that this may be possible at intensities that are still therapeutic.
Taken together, these findings provide an initial basis for evaluating ramp rates for closed-loop DBS. Future studies should expand on this framework to include more sophisticated optimization techniques beyond a binary search and also incorporate more patient behavior. Ultimately, this process enables researchers/clinicians to adequately adjust ramp rates as closed-loop DBS becomes more widely used.
Acknowledgment
The authors would like to thank the participants and members of the Human Motor Control and Neuromodulation Lab. This work was supported by the NIH Grant No: 1UH3NS107709, Parkinson’s Foundation, the Robert and Ruth Halperin Foundation, John A. Blume Foundation, Helen M. Cahill Award for Research in Parkinson’s Disease, and the Stanford Bio-X Graduate Fellowship. Medtronic PLC provided the devices at no charge to the patients but no additional financial support.
Contributor Information
Matthew N. Petrucci, Department of Neurology and Neurological Sciences at Stanford University, Stanford, CA, 94305 USA..
Kevin B. Wilkins, Department of Neurology and Neurological Sciences at Stanford University, Stanford, CA, 94305 USA..
Gerrit C. Orthlieb, Department of Neurology and Neurological Sciences at Stanford University, Stanford, CA, 94305 USA..
Yasmine M. Kehnemouyi, Department of Neurology and Neurological Sciences at Stanford University, Stanford, CA, 94305 USA..
Johanna J. O’Day, Department of Bioengineering at Stanford University, Stanford, CA, 94305 USA..
Jeffrey A. Herron, Department of Neurological Surgery at the University of Washington, Seattle, WA, 98104 USA..
Helen M. Bronte-Stewart, Department of Neurology and Neurological Sciences at Stanford University, Stanford, CA, 94305 USA..
References
- [1].Volkmann J, Moro E, and Pahwa R, “Basic algorithms for the programming of deep brain stimulation in Parkinson’s disease,” Mov Disord, vol. 21 Suppl 1, pp. S284–9, 2006. [DOI] [PubMed] [Google Scholar]
- [2].Koeglsperger T, Palleis C, Hell F, Mehrkens JH, and Bötzel K, “Deep brain stimulation programming for movement disorders: Current concepts and evidence-based strategies,” Front. Neurol, vol. 10, no. MAY, pp. 1–20, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Velisar A et al. , “Dual threshold neural closed loop deep brain stimulation in Parkinson disease patients,” Brain Stimul, vol. 12, no. 4, pp. 868–876, 2019. [DOI] [PubMed] [Google Scholar]
- [4].Little S et al. , “Adaptive deep brain stimulation in advanced Parkinson disease,” Ann. Neurol, vol. 74, no. 3, pp. 449–457, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Arlotti M et al. , “Eight-hours adaptive deep brain stimulation in patients with Parkinson disease,” Neurology, vol. 90, no. 11, pp. e971–e976, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bronte-Stewart HM et al. , “Perspective: Evolution of Control Variables and Policies for Closed-Loop Deep Brain Stimulation for Parkinson’s Disease Using Bidirectional Deep-Brain-Computer Interfaces,” Front. Hum. Neurosci, vol. 14, no. August, pp. 1–8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].O’Day JJ, Kehnemouyi YM, Petrucci MN, Anderson RW, Herron JA, and Bronte-Stewart HM, “Demonstration of Kinematic-Based Closed-loop Deep Brain Stimulation for Mitigating Freezing of Gait in People with Parkinson’s Disease,” Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. EMBS, vol. 2020-July, pp. 3612–3616, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hell F, Palleis C, Mehrkens JH, Koeglsperger T, and Bötzel K, “Deep brain stimulation programming 2.0: Future perspectives for target identification and adaptive closed loop stimulation,” Front. Neurol, vol. 10, no. APR, pp. 1–11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gilron R et al. , “Chronic wireless streaming of invasive neural recordings at home for circuit discovery and adaptive stimulation,” bioRxiv, p. 2020.02.13.948349, Jan. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stanslaski S et al. , “A Chronically Implantable Neural Coprocessor for Investigating the Treatment of Neurological Disorders,” IEEE Trans. Biomed. Circuits Syst, vol. 12, no. 6, pp. 1230–1245, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Afzal MF, Velisar A, Anidi C, Neuville R, Prabhakar V, and Bronte-Stewart H, “Proceedings #61: Subthalamic Neural Closed-loop Deep Brain Stimulation for Bradykinesia in Parkinson’s Disease,” Brain Stimul, vol. 12, no. 4, pp. e152–e154, 2019. [Google Scholar]
- [12].Anidi C et al. , “Neuromodulation targets pathological not physiological beta bursts during gait in Parkinson’s disease,” Neurobiol. Dis, vol. 120, no. May, pp. 107–117, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].O’Day J, Syrkin-Nikolau J, Anidi C, Kidzinski L, Delp S, and Bronte-Stewart H, “The turning and barrier course reveals gait parameters for detecting freezing of gait and measuring the efficacy of deep brain stimulation,” PLoS One, vol. 15, no. 4, pp. 1–17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


