Figure 7. Schematic representation of Atg23 dimerization, membrane binding, and vesicle tethering.
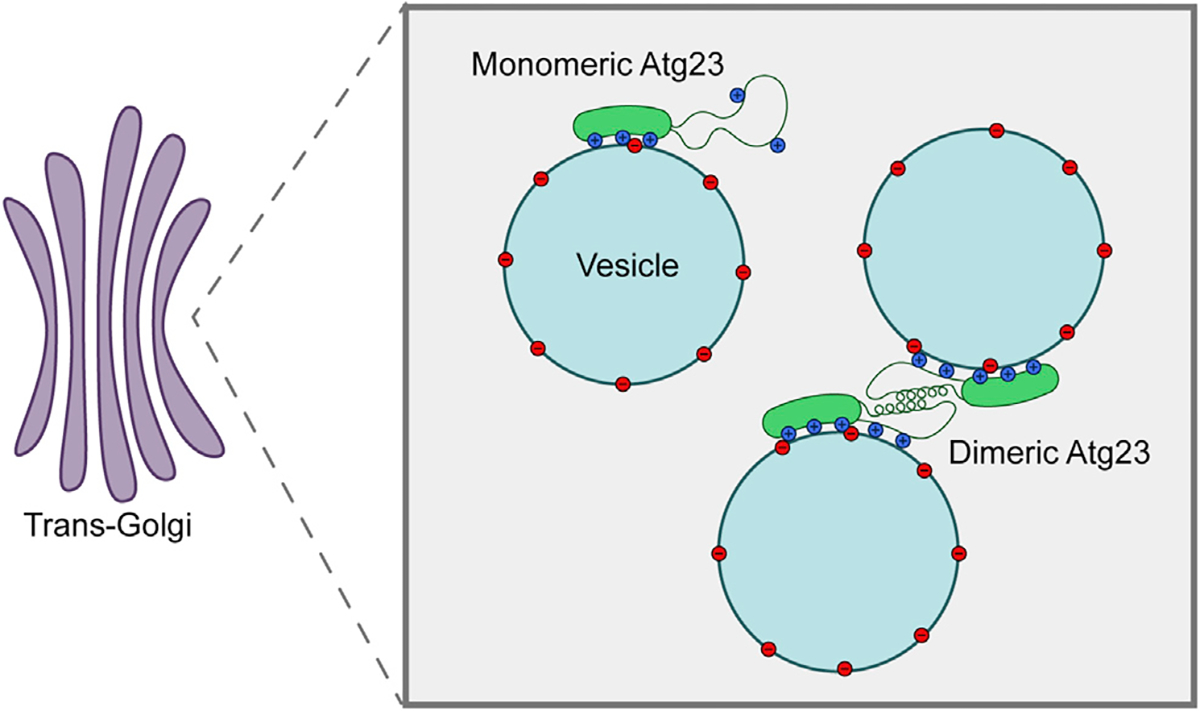
Both monomers of the protein adhere to negatively charged vesicles through several spatially dispersed residues. Dimerization of Atg23 mediated by the putative amphipathic helix results in the tethering of Atg9-containing vesicles at the trans-Golgi. Mutation of key hydrophobic residues within this putative amphipathic helix prevents dimerization as well as proper positioning of some membrane-binding residues. Thus, monomeric Atg23 is entirely deficient in subcellular localization, membrane tethering, and autophagic function while being partially deficient in membrane and Atg9 binding.
