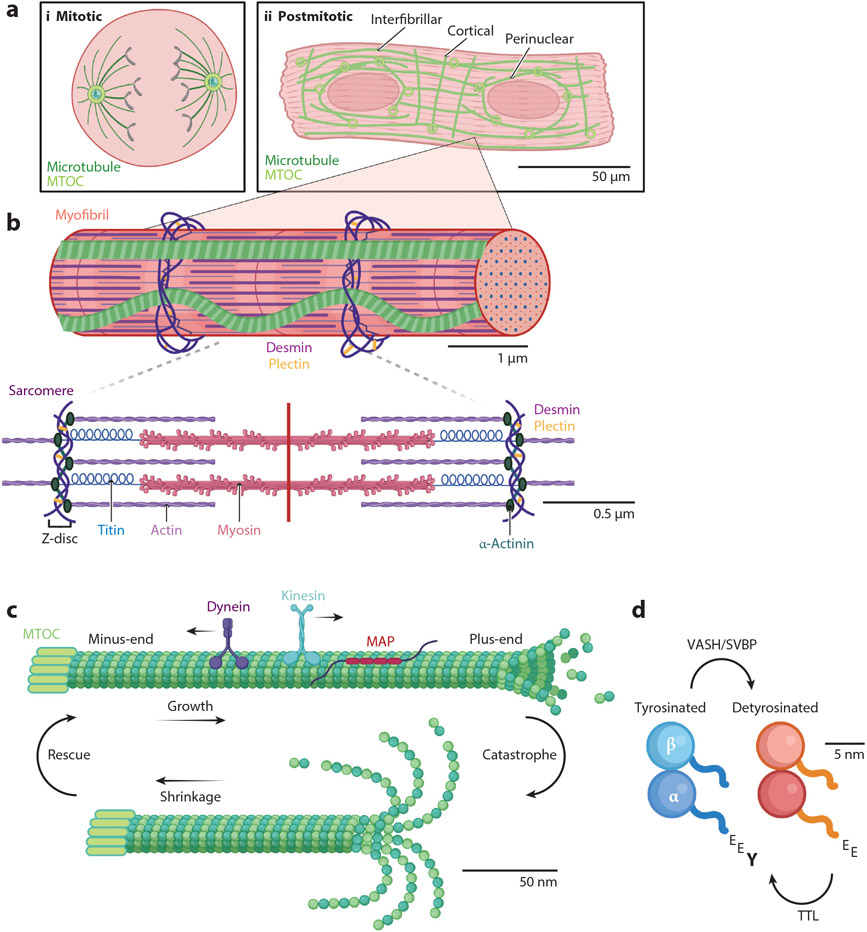
Figure 1.
Microtubules in the cardiomyocyte. (a) Cardiomyocytes transition from mitotic (i) to postmitotic (ii) shortly after birth, changing the localization of microtubule nucleation factors, such as microtubule organizing centers (MTOCs) and, thus, the polarity of the network. Mature cardiomyocytes are comprised of three microtubule populations (all in green): interfibrillar, cortical, and perinuclear. (b) Each myofibril in the cardiomyocyte contains many individual units called the sarcomere. The sarcomere is composed primarily of actin (purple) and myosin (pink). α-Actinin (dark teal) serves to anchor actin at the Z-disc. Desmin (dark purple), cross-linked by plectin (yellow), is also present at the Z-disc and can reinforce microtubules at this location. (c) Microtubules undergo dynamic instability, meaning they can grow or shrink and transition between these modes through rescue and catastrophe. Additionally, the microtubule on top is decorated with microtubule-associated proteins (MAPs; red) and the motor proteins kinesin (blue) and dynein (purple), which step along the microtubule lattice toward the plus-end or minus-end, respectively. (d) One of the most-studied tubulin posttranslational modifications in the cardiomyocyte is detyrosination, the removal of the distal tyrosine on the C-terminal tail of α-tubulin (Y; bottom left). The tyrosination cycle is mediated by the enzymes tubulin tyrosine ligase (TTL), which adds the tyrosine to form tyrosinated tubulin (blue) and vasohibin/small vasohibin-binding protein (VASH/SVBP), which removes the tyrosine to form detyrosinated tubulin (orange).