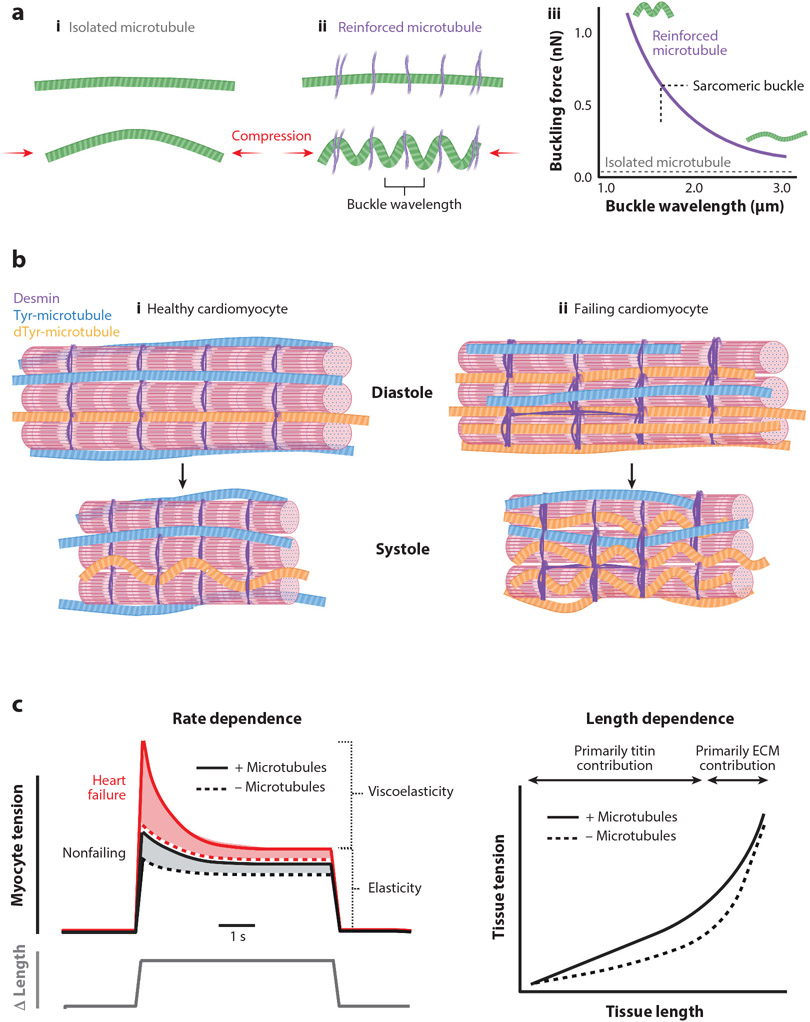
Figure 2.
(a) Microtubule mechanics. (i) Under compression (red arrows), isolated microtubules tend to buckle in one single long arc. (ii) When reinforced by other structural elements in the cell (purple), microtubules tend to buckle between sites of reinforcement, leading to a shortened buckle wavelength. (iii) Isolated microtubules will buckle under very small loads (~1 pN; gray dotted line). When reinforced, increased force is required to cause microtubule buckling, with higher forces leading to shorter buckling wavelengths. In the cardiomyocyte, microtubules tend to buckle with a wavelength of ~1.65 μm, the length of a sarcomere in systole (black dotted line). (b) Microtubules during myocyte shortening. Healthy cardiomyocytes (i) have a mix of tyrosinated (Tyr) microtubules (blue) and detyrosinated (dTyr) microtubules (orange). During systole (bottom), the laterally anchored detyrosinated microtubules buckle and provide a small resistive element against myocyte motion. In failing cardiomyocytes (ii), there is an increase in the number of stable detyrosinated microtubules. Additionally, there are more desmin intermediate filaments (purple) that provide lateral stabilization to detyrosinated microtubules. Together, these modifications result in more buckling and resistance to contraction during systole and relengthening during diastole, contributing to contractile dysfunction. (c) Microtubules during myocyte stretch. (Left) Rate dependence. In response to a rapid increase in strain (bottom line), there is a transient increase in tension (top line) in nonfailing (black) cardiomyocytes that dissipates to a steady-state level slowly over time. The transient component reflects the viscoelastic resistance to stretch, while the steady-state component reflects the elastic resistance. In cardiomyocytes from failing hearts (red), the viscoelastic contribution to tension is exacerbated. In the absence of microtubules (dotted lines), the viscoelastic component is largely diminished, while the elastic component is largely unaffected. (Right) Microtubule length dependence. Microtubules (solid black line) cause pathologically remodeled myocardium to resist stretch and increase tissue tension more than in myocardium with disrupted microtubules (dotted black line). At high strains, the traces converge as the tissue tension is largely dominated by the contribution of the extracellular matrix (ECM). The viscoelastic resistance imposed on cardiomyocytes and myocardium by detyrosinated microtubules contributes to impaired diastolic function in failing hearts.