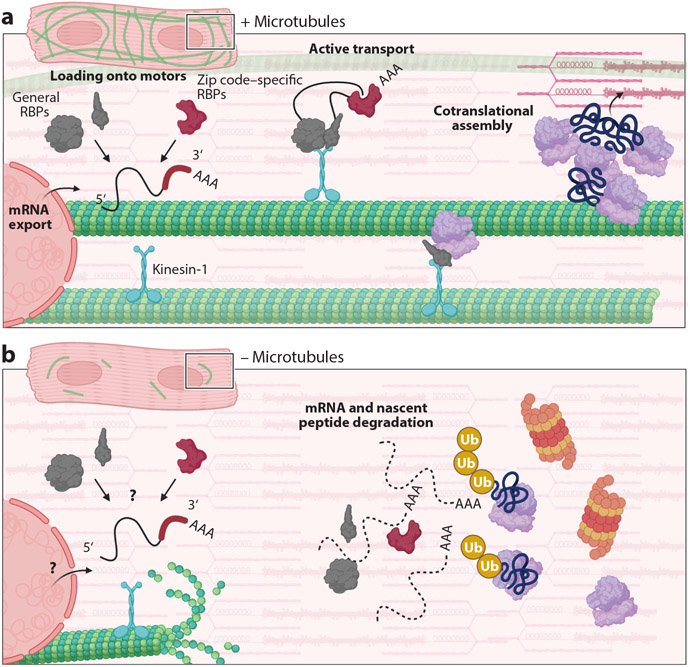
Figure 3.
mRNA transport in the cardiomyocyte. (a) Cardiomyocytes with an intact microtubule network export mature mRNA (as indicated by the polyA tail, …AAA) that forms a complex with a variety of general RNA-binding proteins (RBPs; gray) and zip code–specific RBPs (red), forming a heterogeneous ribonucleoprotein complex to be loaded onto kinesin-1 (blue). This particle is then actively transported on the microtubule. Ribosome subunits (purple) are also actively transported via motors. mRNAs and ribosomes are trafficked to both the Z-disc and intercalated disc to form a translational hub (top right). Here, sarcomeric mRNAs are cotranslated to facilitate assembly of structures and incorporation into existing sarcomeres. (b) In the absence of microtubules, both mRNA and ribosomes are mislocalized in the cardiomyocyte. It is unknown whether mRNA export, and/or the assembly of RBPs onto the mRNA, is inhibited in this scenario (bottom left). This perinuclear collapse of mRNA results in mislocalized translation and the degradation of mRNA and new peptides (indicated by addition of ubiquitin, Ub, to peptides; bottom right). Under these circumstances, the cardiomyocyte is unable to grow due to its inability to localize and add new sarcomeric protein.