FIGURE 1.
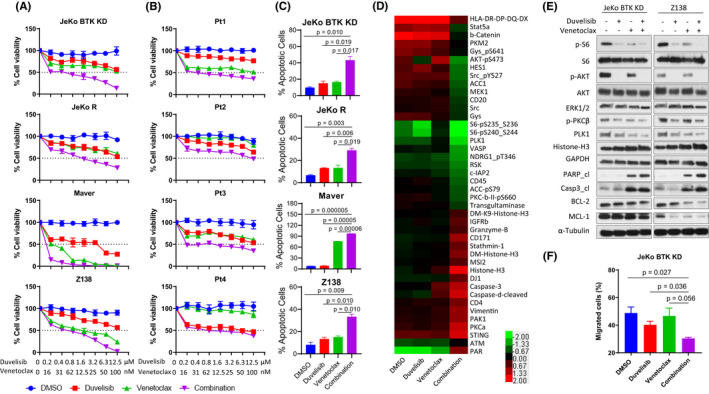
Duvelisib and venetoclax in combination synergistically reduced cell growth and induced apoptosis in MCL. (A, B) Four IR MCL cell lines (A) and four patient samples (B) were treated with DMSO, increasing doses of duvelisib (0–12.5 μM), venetoclax (0–100 nM) and the combination. Cell viability was detected at 72 h (A) or 24 h (B). (C) Four MCL IR cell lines were treated with vehicle, duvelisib (5 μM), venetoclax (100 nM for JeKo BTK KD and JeKo R, or 10 nM for Maver and Z138) and the combination. Cell apoptosis was determined by annexin‐V/PI staining, and both annexin‐V+/PI− and annexin‐V+/PI+ subpopulations were considered apoptotic cells. Each treatment for cell viability and apoptosis was set up in triplicate and repeated at least two independent times. (D) 5 × 106 JeKo BTK KD cells were treated with vehicle, 5 μM duvelisib, 100 nM venetoclax, or the combination for 24 h and harvested for RPPA analysis. Proteins with more than a twofold change between the combination and vehicle control were selected for heatmap generation using Cluster 3.0 and Java Treeview. Each treatment for RPPA was set up in triplicate. (E) Two MCL IR cell lines, JeKo BTK KD and Z‐138, followed the same treatments as RPPA analysis and protein lysates were collected for Western blotting. (F) CMFDA‐labelled JeKo BTK KD cells were pre‐treated for 30 min with either vehicle, 5 μM duvelisib or 100 nM venetoclax, alone and in combination before loading to the upper chamber of the Transwell migration system. The bottom chamber was pre‐seeded with CMFDA‐unlabelled HS‐5 monolayer overnight. At 4 h of incubation, the cell counts of CMFDA‐positive MCL cells migrated to the bottom chamber were determined by flow cytometry and total cell counts in the bottom. The percentage of CMFDA‐labelled MCL cells that migrated into the lower chamber out of total cells loaded into upper chamber were generated and plotted
