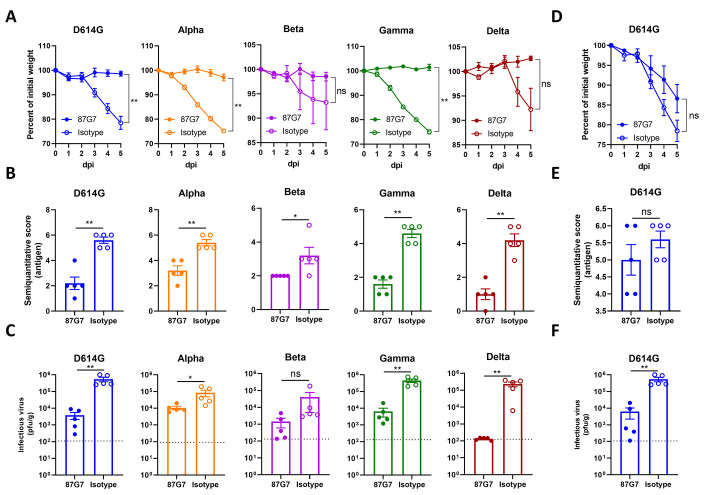
Fig. 4. 87G7 protects mice from challenge with D614G SARS-CoV-2 and Alpha, Beta, Gamma or Delta variants.
Prophylactic and therapeutic treatment was assessed in the K18-hACE2 SARS-CoV-2 mouse model. 87G7 or isotype control mAb was administered intraperitoneally (10 mg/kg body weight) into groups of mice (n = 5) at 24 hours before (A, B, C) or after virus challenge (D, E, F). Mice were challenged intranasally with 105 PFU of SARS-CoV-2 (D614G, Alpha, Beta, Gamma or Delta) and monitored daily for weight loss (A and D). Five days after challenge lungs were collected from all mice, and lung viral antigen levels were determined by immunohistochemistry (B and E; Table S2, Figure S6), and infectious SARS-CoV-2 loads in lung tissue were measured by plaque assay (C and F). The mean values ± SEM of all data points were shown. Dashed line indicates assay limits of detection. Mann-Whitney U test was used to evaluate the statistical difference between the 87G7 and isotype-treated groups (**p<0.01, *p<0.05, ns p>0.05).