Figure 4. Subgroup analyses of five drug cohort studies to evaluate confounding by disease comorbidities.
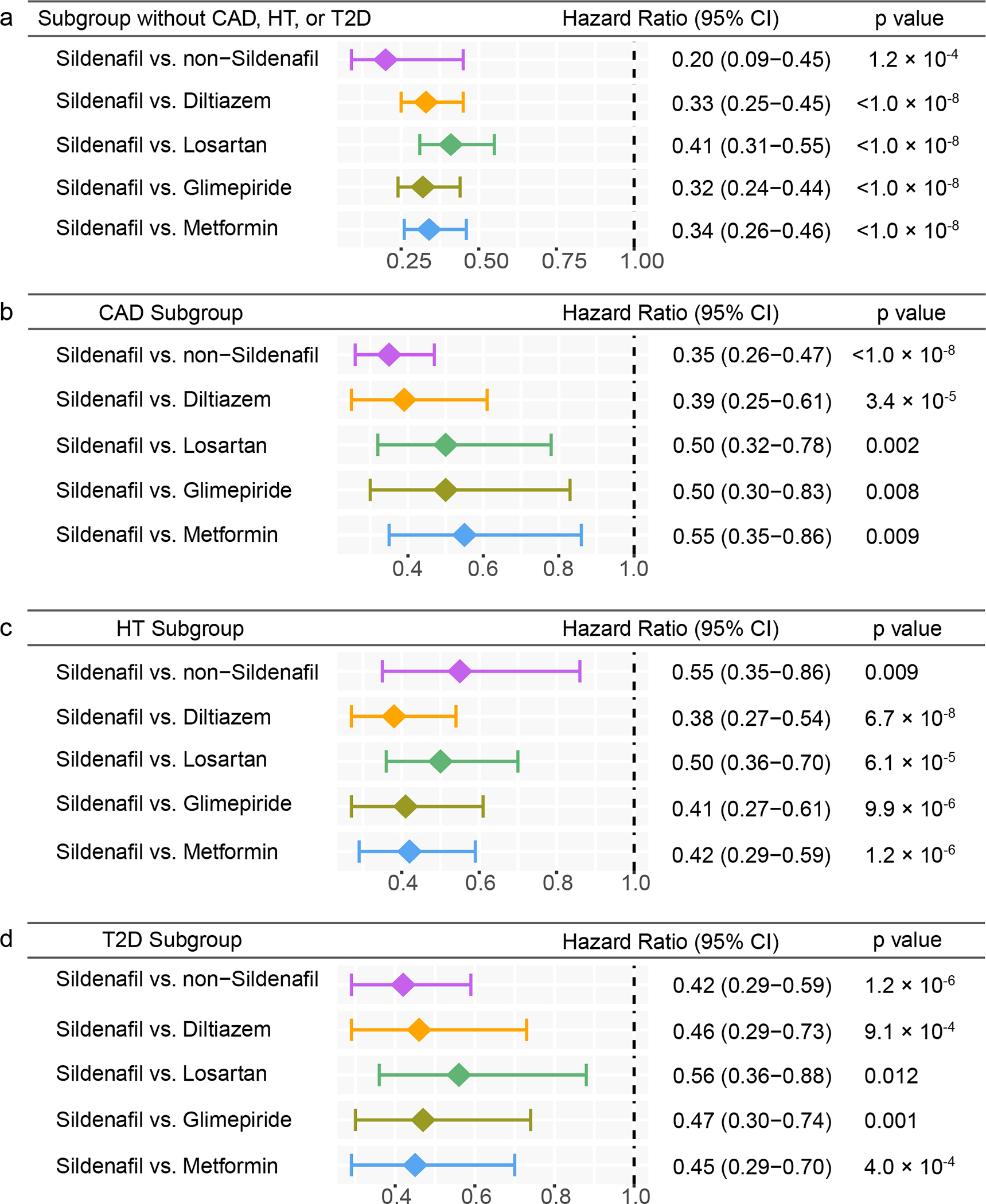
(a) Hazard ratios (HR) and 95% confidence intervals (CI) across five cohort studies after exclusion of individuals with coronary artery disease (CAD), hypertension (HT), and type-2 diabetes (T2D) (sildenafil n = 56,518; diltiazem n = 113,600; losartan n = 275,116; glimepiride n = 52,623; metformin n = 303,008). (b-d) HR and 95% CI plots across five cohort studies in individuals with CAD (b) (sildenafil n = 19,093; diltiazem n = 51,771; losartan n = 111,592; glimepiride n = 30,083; metformin n = 91,705), HT (c) (sildenafil n = 49,541; diltiazem n = 119,097; losartan n = 339,940; glimepiride n = 74,018; metformin n = 275,328), or T2D (d) (sildenafil n = 21,978; diltiazem n = 51,300; losartan n = 156,308; glimepiride n = 100,298; metformin n = 367,754). Non-sildenafil exposure population were matched to the exposures (ratio 4:1) by adjusting the initiation time of sildenafil, enrollment history, sex, and disease comorbidities (CAD, T2D, and HT). Propensity score stratified Cox-proportional hazards models and two-sided log-rank test were used to conduct statistical inference for the hazard ratios.
