Abstract
BACKGROUND:
Despite known benefits, palliative care consultation for hospitalized patients remains underutilized and lacks consistency across disease type.
OBJECTIVE:
To improve the frequency and timeliness of appropriate inpatient palliative care consultation.
DESIGN:
On 2 of 11 hospitalist teams, a palliative care social worker and physician attended multidisciplinary discharge rounds twice a week. Control teams’ discharge rounds were unenhanced.
SETTING/SUBJECTS:
All patients admitted to a hospitalist service in an 800-bed quaternary academic medical center in the United States.
MEASUREMENTS:
Primary outcome: change in provision of palliative care consultation over time (in absolute percentage-points, using concurrent and historic control patients, measured as a difference-in-differences (DID) in outcomes), using logistic regression to control for random-effects based on hospitalist physician. Secondary outcome: change in time-to-consult (days) using parametric survival analysis of median days to achieve consult in 5% of patients. Hospitalists were surveyed regarding perceived effectiveness and clinical burden.
RESULTS:
Adjusting for multiple covariates, the intervention increased palliative care consultation (DID=1.0 percentage-point increase [95% CI 0.3–1.8%]) and decreased time to consult (DID=−5 days [95% CI −11 to −1]) in patients admitted for non-cancer diagnoses, but did not increase palliative care consultation (DID −2.1 percentage point decrease, 95% CI −.07, +.03) or decrease time-to-consult (DID 1, 95% CI −5 days to + 8 days) for patients admitted with cancer diagnoses. Hospitalists thought the intervention facilitated effective patient care without increased burden.
CONCLUSIONS:
An interprofessional intervention improved utilization of palliative care expertise for non-cancer patients without additional burden to hospitalists.
Keywords: Palliative Care, Interprofessional, Improved Utilization
INTRODUCTION
The care of patients at the end of life (EOL) is of growing concern in medicine.1 Palliative care (PC), which focuses on quality of life and symptom relief for patients with serious illness, has been shown to extend life expectancy,2,3 and increase satisfaction with care.4,5 PC use also decreases costs,6–8 readmissions,9 and reduces patient symptoms.5,10,11 However, PC expertise remains a limited resource. Expanded access to PC cannot solely rely on specialists providing direct care for all patients with serious illness.12 Despite rates of inpatient PC consultations increasing,13 hospitalized patients do not receive PC services as often or as soon as would be beneficial.14–16
While integration of PC into various settings, such as oncology and surgery, has resulted in greater utilization and earlier access, PC utilization is not uniform.17,18 Quality of EOL care is poorer among patients with organ failure and frailty, in comparison to end-stage dementia and cancer, as a result of receiving fewer PC consults.19 Although disease and critical care specific screening tools exist, they may not be applicable to a heterogenous general care inpatient population.20 Innovative strategies are required to maximize appropriate use of PC resources and ensure that patients who may benefit have an opportunity to receive PC services.21 To address this need, we initiated a novel interprofessional intervention with co-rounding PC specialists and hospitalists to identify inpatients for appropriate PC utilization and determine impact on time to consultation. We also explored whether the intervention would have more impact on non-cancer patients, who historically have limited access to PC interventions.19
METHODS
Design & Setting
This study took place at Michigan Medicine, an 800-adult bed quaternary care academic medical center. Study patients were cared for on the hospitalist service consisting of 11 teams, each staffed by an attending hospitalist. Given the complexity of the patient population and the team consisting of an attending physician without mid-level providers or residents, each team is capped at 11 patients. Usual care on this service includes daily discharge rounds (Monday-Friday) to discuss hospitalization goals and anticipated discharge needs for each patient. Prior to our intervention, PC consultation was typically requested ad hoc by hospitalists in reaction to patients’ symptoms or need for complex goals of care discussions.
In this study, we aimed to proactively identify patients to facilitate timely and appropriate PC consults. The intervention started 7/1/2013 and ended 6/30/2014. We used a non-randomized intervention (IRB HUM00078475) design with concurrent and historic controls to capture a difference in change over time. Coincident to the intervention initiation, the capacity of the PC consultation service doubled from one to two consultation teams, potentially confounding results of a purely pre-post study design. Therefore, we enhanced the strength of our pre-post study by maintaining a non-intervention control group and measuring the differences-in-differences (DID)22 to determine the effect of the intervention. This method 22 compares pre- and post- data between the control versus intervention groups to analyze differences in the change of a proportion over time. The data is analyzed as a logistic regression, with intervention versus control group as a the primary predictor variable, and pre- vs post- time period indicator variable as a second predictor, and the interaction between the two variables to evaluate the statistical significance of the DID.
Patient Sample & Data
Data for all patients admitted to the 11 hospitalist teams from 7/1/ 2012 through 6/30/2014 was obtained in 2015. Each inpatient admission was considered to be the primary unit of analysis. Administrative data including gender, age, admission date, discharge date, death date if applicable, all submitted claims for procedures and physician visits, conditions, and the diagnosis-related group (DRGs) associated with the hospitalization were sourced from administrative hospital data.
Intervention
Two of the eleven hospitalist teams were randomly assigned to participate in the intervention. The remaining nine teams were considered controls. No changes were made to their work process. The intervention consisted of enhancing the scope of the discharge rounds by adding the attending and social worker from the PC team twice weekly. During enhanced rounds, all patients currently on the hospitalist’s service were discussed. PC consultants spent approximately 15 minutes of time per team meeting. If appropriate, the consultant offered informal recommendations concerning whether a patient would benefit from formal inpatient consultation. PC providers utilized clinical judgement to make recommendations about which patients could benefit from PC services, rather than using a specific screening tool. Recommendations ranged from symptom management to goals of care. The recommendation to obtain a consult was not necessarily a self-referral, new PC consults are distributed across the two PC consultation teams through an intake pager, and in all cases hospitalists made the final determination whether to pursue an inpatient consultation.
Following the intervention period, participating hospitalists (n=25) were electronically surveyed to elicit perceived value and/or burden of the intervention, and change in utilization of PC services. Survey questions consisted of seven items; two assessing hospitalists’ time commitment related to the intervention, four matrix tables assessing utilization with 5–10 statements rated on a 5-point Likert scale (strongly disagree to strongly agree; never to always), and one free-text question inviting respondents to share any additional thoughts about their experience.
Measurement
This was an intent-to-treat study. Patients were considered as having received the intervention if they were admitted to an intervention team, regardless of whether or not they were discussed during the enhanced discharge rounds. To determine which patients were on an intervention team or not, we matched the doctor providing medical care (available from hospital administrative billing data) on each day of hospital care to the attending hospitalists’ schedules for each of the hospitalist teams. Two of the hospitalist teams were considered to be intervention teams, the nine remaining teams were control. Team assignment was based on the modal physician who provided daily hospital care, with priority to the team on the day of discharge if there was a discrepancy. For a small number of patients, no clear hospitalist team could be identified due to insufficient contact and thus excluded. These patients were either: (1) never assigned a team due to overnight admission by a float physician and morning discharge (including death), or (2) immediately transferred to another service (e.g., intensive care unit) and never returned to the hospitalist service. Using a random sample of 200 patients, we reviewed the medical record blinded to our administratively-assigned teams to determine the true hospitalist team. Agreement on intervention versus control team assignment was 96.8% with a kappa of .78.
The primary outcome was change over time in proportion of admitted patients receiving a PC consult. The change over time was measured as the absolute difference in percent of hospitalizations during the intervention year (2014) minus the prior year (2013). For this reason, the hospitalization was considered to be the primary unit of analysis, with a new opportunity for PC consultation once a patient was discharged and readmitted. We first linked the PC attending physicians’ schedules to professional claims for inpatient consultations. Patients seen or discussed by members of the PC team without a physician to bill for consultation were not captured in the administrative data as a consult.
The secondary outcome of time to consultation was calculated as the time between date of admission to the date of the first PC consultation. Using a random sample of 200 hospitalizations, half with a known PC consultation, we independently reviewed the medical record blinded to status of PC consultation. Accuracy of occurrence of a PC consult was 98.9% agreement with a kappa of .98, and exact date of the initial palliative consult visit agreement was 96.4% with a kappa of .96.
Because our intervention was non-randomized, we controlled for age, gender, readmissions, Charlson comorbidity score (CCS),23 length of stay (LOS) and Relative Value Units (RVUs). Because some patients were repeatedly hospitalized, potentially increasing the motivation for PC consultation, we also classified patients’ hospitalization as being a readmission if the patient had been discharged from any service in the system within the past 30 days. A hospitalization was considered cancer-related if the primary DRG was for a solid tumor, leukemia, lymphoma, or metastatic cancer.24 RVUs were determined as a sum of RVUs associated with all billed Common Procedural Terminology (CPT) codes for procedures and evaluation and management across the duration of the hospitalization, with a higher RVU measure indicating a greater total provision of hospital services. There were no missing co-variable data.
Analysis
Hospitalist survey results were analyzed using descriptive statistics. For patient data, we first compared baseline characteristics between the intervention versus control groups to test for comparability using one-way analysis of variance for continuous variables (age, RVUs LOS, CCS) and chi-squared tests for proportions (gender, cancer diagnosis, readmission, and hospital death).
Our primary analysis compared the change in proportion receiving a PC consult (present versus absent) between the intervention and control groups over time using a DID model. Because our outcome was non-linear (binomial), the resulting odds ratio and confidence interval does not directly result in the effect of the intervention, as in linear regression.25 Therefore, we obtained the DID (and 95% confidence intervals) by bootstrapping 1000 repetitions to obtain 50th, 95th and 2.5th observations, holding continuous control variables by their means and categorical variables by their mode. By chance, cancer admissions (a strong predictor of PC consultations 17) was substantially more prevalent in the intervention teams. Therefore, all multivariable analyses considered cancer as both a control variable and interaction term, allowing for a differential intervention effect among cancer versus non-cancer patients. Because the effect of the intervention might have disproportionately affected some hospitalists more than others, we included a random effect by physician.
Second, we compared time to consultation between intervention and control groups. Patients were censored upon discharge. We visually inspected Kaplan-Meier curves to ensure similar hazard functions, followed by a Cox proportional hazard model. Then, we estimated the DID in days-to-consultation, using parametric survival analysis. In survival analysis of time-to-death, median life expectancy describes the time over which half the population have died. However, in this study, consultation occurred in much fewer than half of the patients, so the traditional median time-to-consult greatly exceeds a typical hospital stay and introduces unacceptable uncertainty. Therefore, to meaningfully report the DID associated with the intervention, we based results on an earlier time point, the median time to achieve PC consultation in 5% rather than 50% of the population. We controlled for the same factors as in the primary analysis except LOS (omitted due to correlation with exposure time) and expressed utilization as average intensity of utilization per day rather than over the total hospitalization (which would also be correlated with exposure time). To perform the parametric analysis, we used a log-logarithmic baseline cumulative hazard distribution, which had the lowest Aikake’s criterion. Similar to the primary outcome, we bootstrapped 95% confidence intervals around time-to-consult and the DID. We used Stata 14 for all analyses.25
RESULTS
Patient Characteristics
A total of 15,423 patients were hospitalized during the two-year interval examined. Of these, 1941 (12.6%) had insufficient contact on a hospitalist service, resulting in 13,941 hospitalizations retained for analysis (Figure 1).
Figure 1.
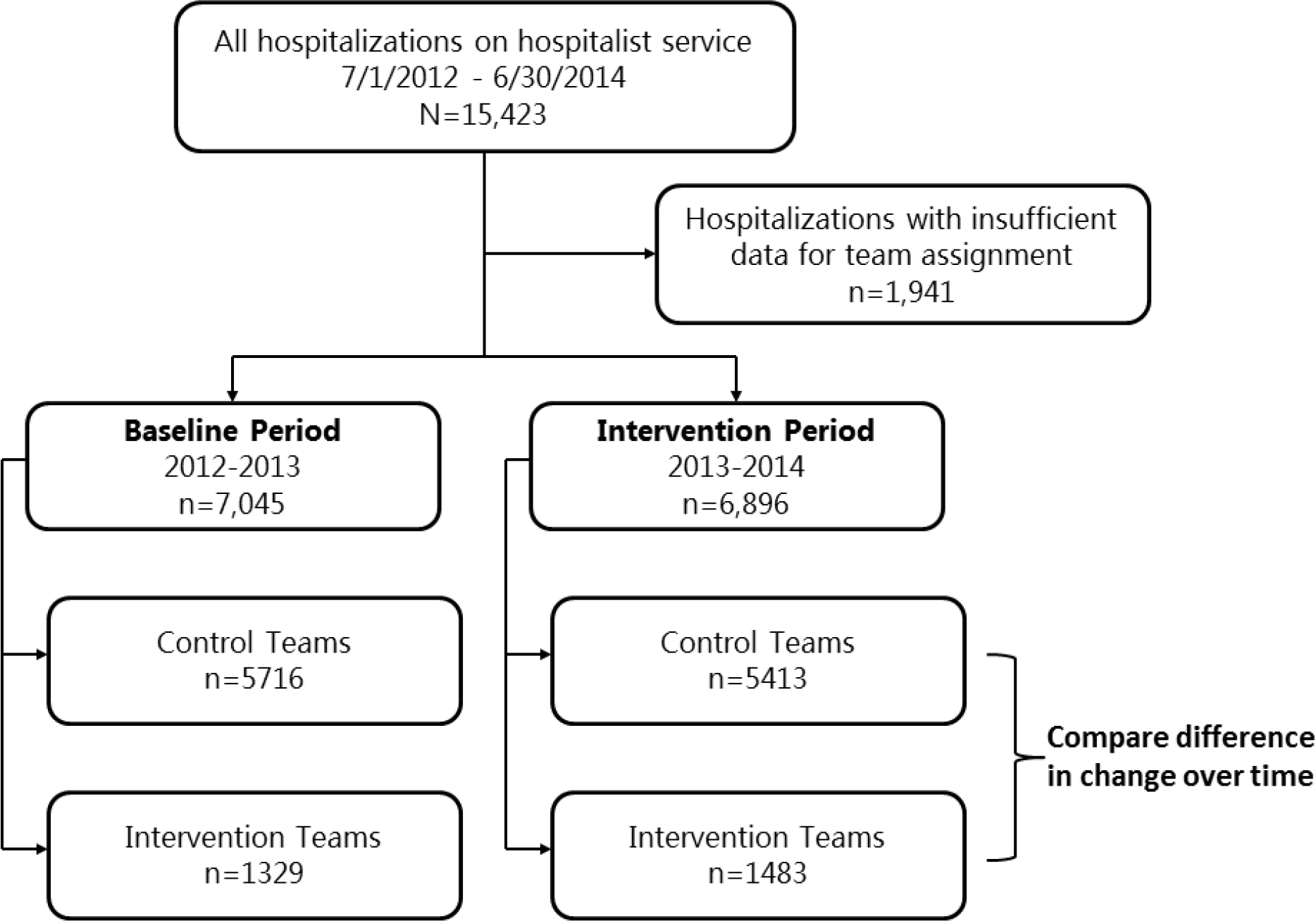
Flow of Data into Difference-in-Differences evaluation
Patient and hospitalization characteristics were similar across intervention and control groups with no significant differences in age, gender, LOS, in-hospital deaths, and readmissions. There were more hospitalizations with cancer diagnosis during the intervention time period on the participating intervention teams (3.8% vs 5.5%, p =.004; Table 1).
Table 1.
Comparison of Patient and Hospitalization Characteristics between Intervention and Control Team Patients during Intervention Phase
| N=13941 | Control patients (n=5413 patients) n(%) or M±SD |
Intervention Group (n=1483) n(%) or M±SD |
p-value |
|---|---|---|---|
| Age | 58.3 ± 18.4 | 57.6 ± 19.0 | 0.151 |
| Male | 2851 (52.7) | 780 (52.6) | 0.960 |
| RVUs | 50.3 ± 55.5 | 50.7 ± 68.5 | 0.851 |
| CCS | 3.0 ± 2.7 | 2.9 ± 2.7 | 0.095 |
| Prior 30-Day Admission | 1009 (18.6) | 268 (18.1) | 0.617 |
| Length of Stay | 5.6 ± 6.4 | 5.7 ± 7.4 | 0.792 |
| Died in Hospital | 98 (1.8) | 26 (1.8) | 0.883 |
| Cancer | 207 (3.8) | 82 (5.5) | 0.004 |
RVUs: total hospital care and resource utilization (tests, procedures, services, professional billing), expressed as relative value units associated with submitted charges over the hospitalization
Readmitted patient: Admission date (for this hospitalization) was within 30 days of an inpatient discharge within this healthcare system.
CCS=Charlson Co-morbidity Score
Results: Proportion Receiving Palliative Care Consultation
Univariate analyses
Of 13,941 hospitalizations in the analytic sample, 392 (2.8%) were provided a PC consult. For the intervention teams, the unadjusted proportion receiving PC consultation prior to the intervention (in 2013) was 2.7%, which increased to 5.2% after implementation of the multidisciplinary team intervention (in 2014, p<.05). The control teams not receiving the intervention during the concurrent time were 3.03% (p< .05 comparing intervention vs control).
To examine the secular change of PC utilization over time, we examined only control teams. PC consultation increased from 2.0% in the pre-intervention time period to 3.0% in the post-intervention year (p<.05).
Multivariate Analyses
Clustering by physician and adjusting for age, gender, prior 30-day admission, LOS, comorbidity, RVUs, cancer-related hospitalization, the predicted proportion receiving a PC consult increased among non-cancer hospitalizations (n=13,356) by 1.38% over the study period for the intervention patients, compared to a 0.38% increased proportion receiving consults among the control patients. This yielded a 1.01% [95% CI 0.3%–1.8%] DID (net change) between the two groups (Figure 2).
Figure 2. Intervention Effect on Palliative Care Consultation in Non-Cancer Patients (DID) (N=13356 Non-Cancer Hospitalizations).
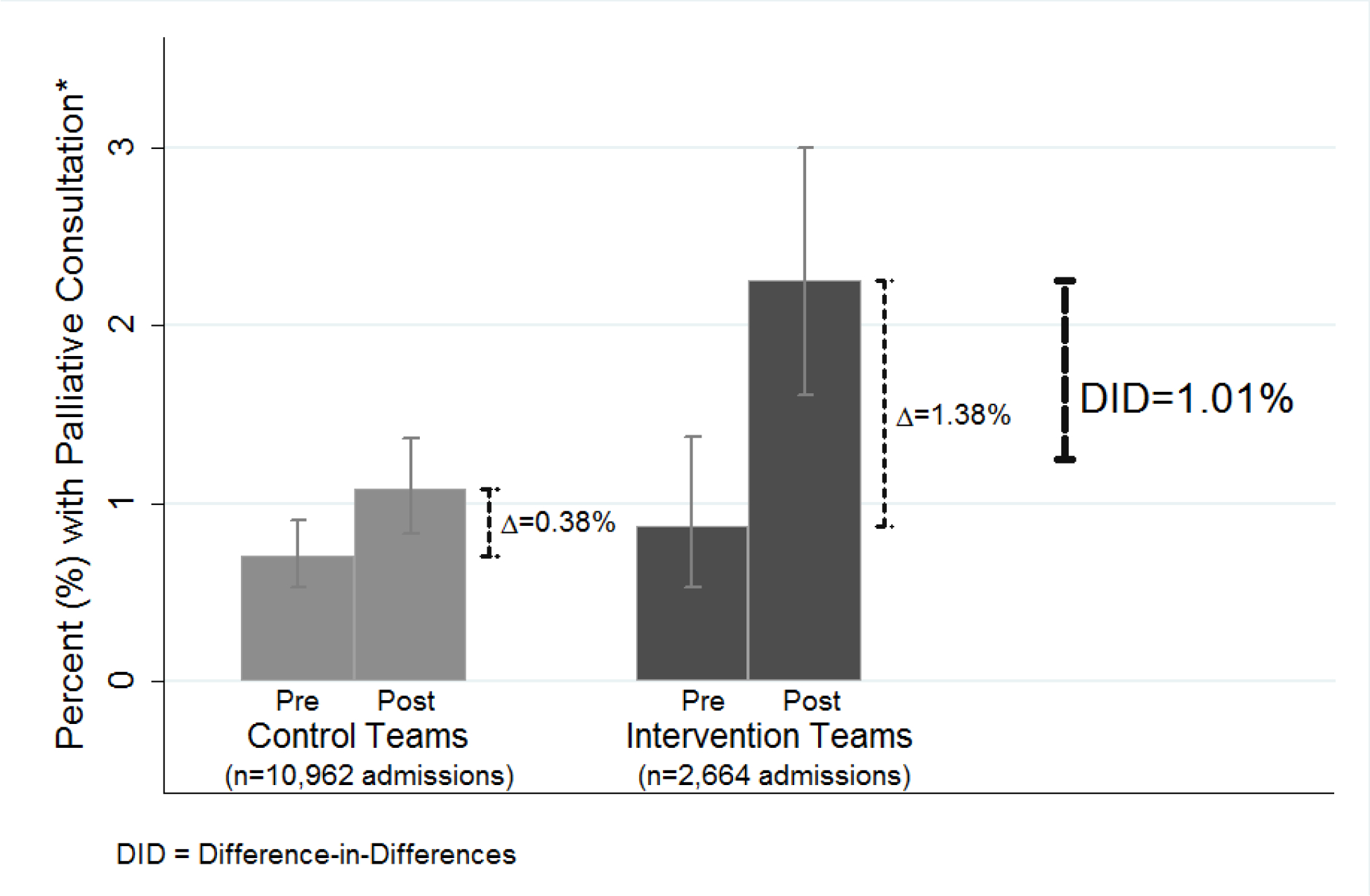
DID = Difference in Differences
The predicted percentages illustrating the DID for a non-cancer patient are based on fully-interacted model of intervention versus control, post- versus pre-intervention time, and cancer versus non-cancer patient. The model also includes a random-effect for physician and multiple control variables, which are held constant at their mean or modal values: a 60-year old male, with 50 total relative value units (RVUs) charged over the hospitalization, Charlson comorbidity score of 3 points, length of stay of 6 days, and no 30-day recent hospitalization.
Comparatively, among cancer patients (n=585), there was no increase attributable to the intervention, with a DID of −2.09% [−0.07, +0.03].
Results: Time to Palliative Care Consultation
Using time to consult as the outcome, the unadjusted HR associated with the post-intervention study versus control team for all patients was 1.74 [1.33–2.29]. The cumulative proportion of patients receiving a consultation (Figure 3) demonstrates the least time to consult in the post-intervention study group. Controlling for all covariates, time-to-consult to achieve 5% consult was reduced by the intervention in non-cancer patients: intervention teams decreased from 20 to 12 days (change = −8 days [−14, −5]), control teams decreased from 20 to 17 (change = −3 days [−6, −1]), resulting in DID = 5-day decrease in time-to-consult [−11 to −1 days] (Figure 4). For cancer patients, the intervention did not affect the days-to-consult for intervention (from 13 to 10 day change = −3 days [−9, −3]), nor control teams (from 12 to 8 days change = −4 days [−8, −1]), resulting in a non-significant DID = +1 day [−5 to 8 days].
Figure 3. Intervention Effect on Cumulative Time to Palliative Consult.
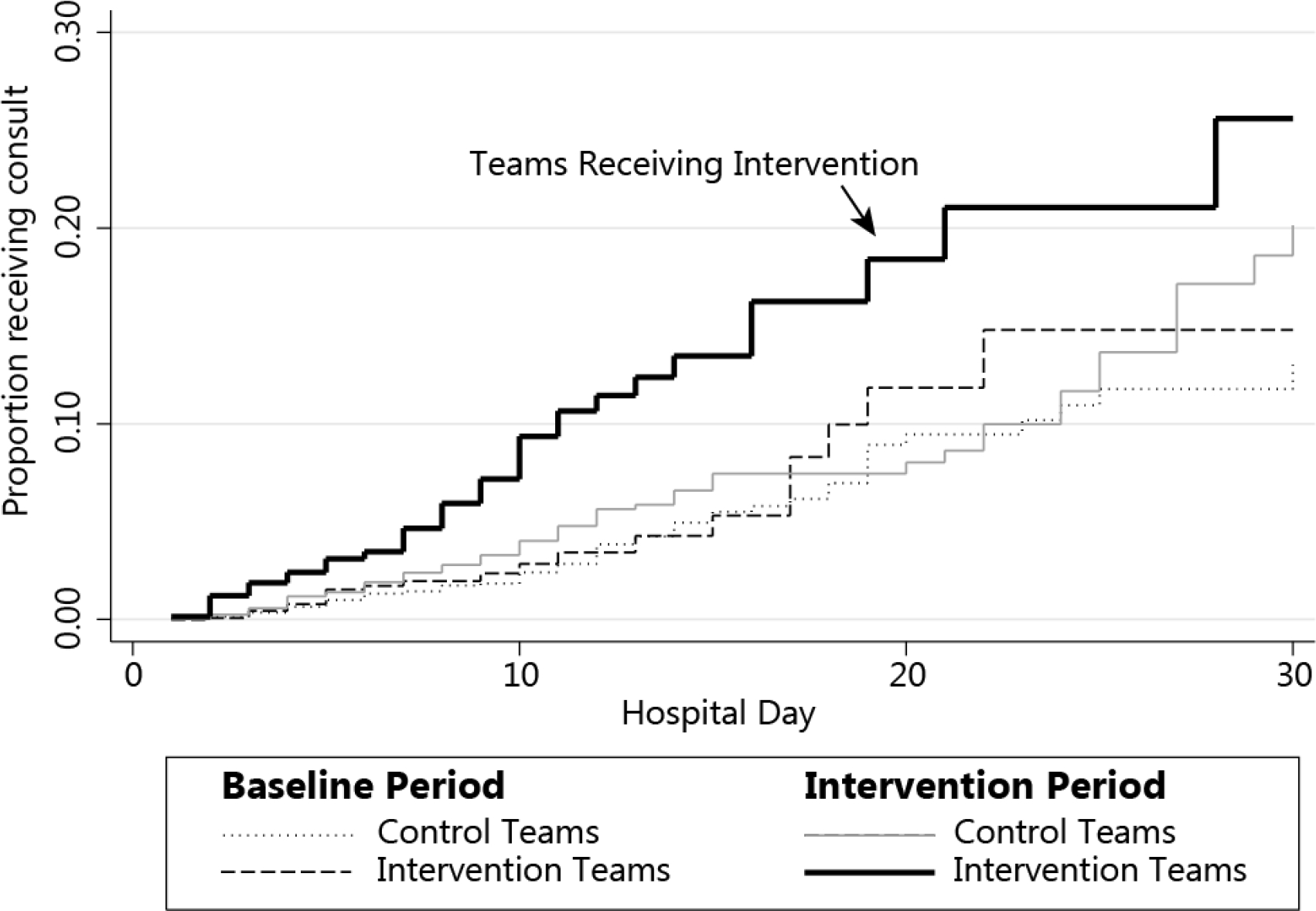
Kaplan-Meier curves describing cumulative proportion of remaining hospital patients receiving Palliative Care consultation, by intervention vs control teams and intervention vs control periods, with the solid black line representing intervention teams during the intervention time.
Figure 4. Intervention Effect on Median Time to Palliative Consult (DID) (N=13356 Non-Cancer Hospitalizations).
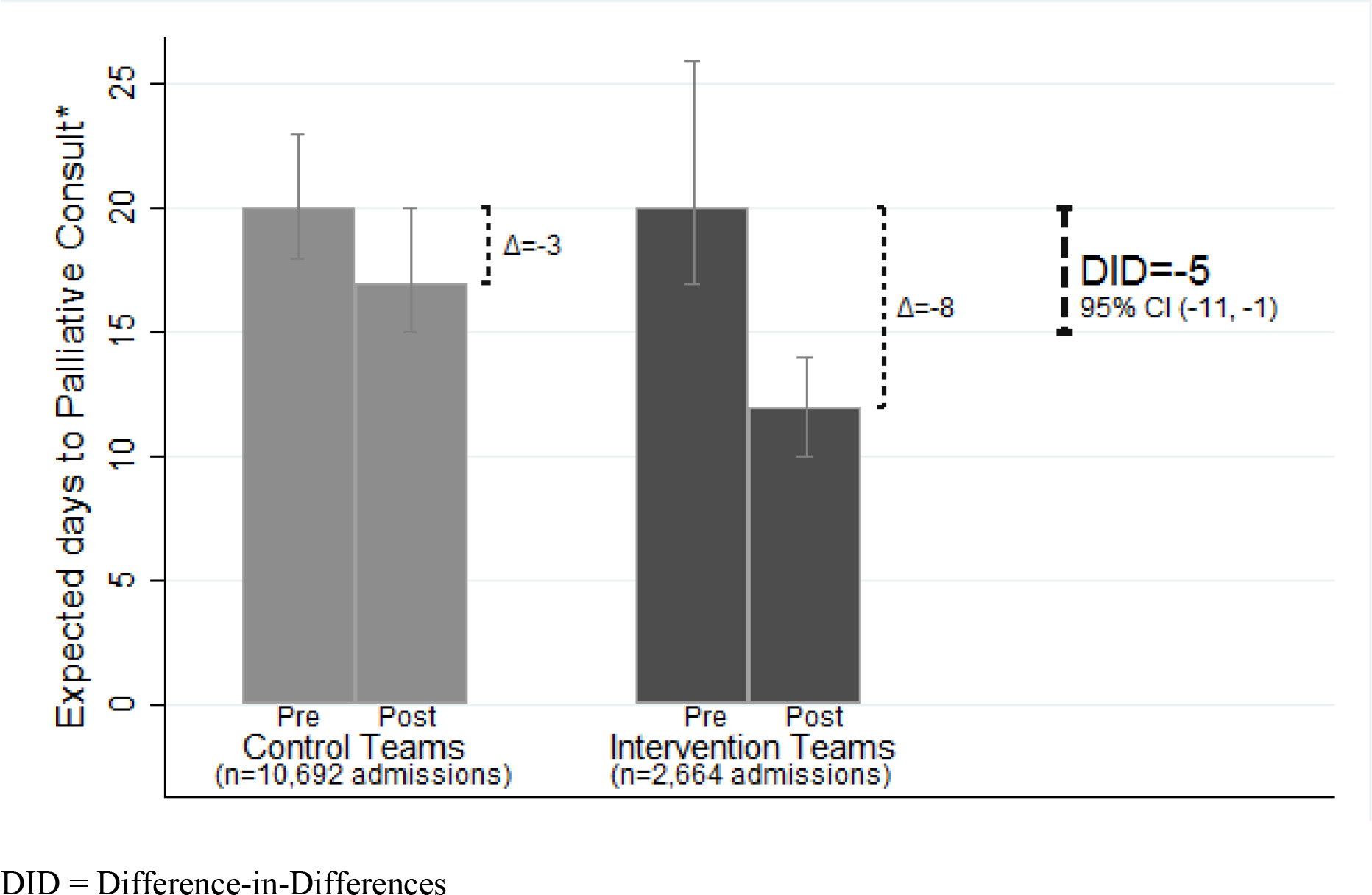
*Days to Achieve Palliative Care Consultation in 5% of admitted non-cancer patients
The predicted percentages illustrating the DID for a non-cancer patient are based on fully-interacted survival model of intervention versus control, post- versus pre-intervention time, and cancer versus non-cancer patients, with covariables held constant at mean or modal values: a 58-year old male patient, with 8 relative value units (RVUs) charged per day, Charlson comorbidity score of 3 points, and no 30-day recent hospitalization.
Results: Survey Responses
Fifteen of the 25 hospitalists participating in the intervention completed the survey (60% response rate). All respondent hospitalists reported that the intervention facilitated easier communication with PC consultants, with 83% agreeing it added value to patient care. Only one hospitalist (out of 15) found the intervention to be too time consuming, while 55% said the intervention improved their own PC skills.
DISCUSSION
Our novel intervention of integrated interprofessional rounding resulted in new PC consultation for 1% of all admitted non-cancer patients - consultations that would not have occurred in the absence of the intervention. Generalizing this result to a large hospitalist service with over 10,000 non-cancer admissions per year, we would have generated 100 new appropriate PC consults for non-cancer patients with this intervention. In addition, this intervention decreased the time-to-consult by 5 days.
This short-term, minimally resource-intensive intervention strategy represents a potential new educational and service paradigm that could extend PC expertise within the health system. While specific screening tools have been shown to lead to earlier PC consultation in ICU settings, the Improving PC in the ICU Advisory Board acknowledges that in some settings other strategies may be more appropriate.21 In contrast to formal screening tools, we identified appropriate patients in a focused and individualized fashion, tailoring our intervention to meet the needs of a heterogenous general care patient population. We hypothesize that this intervention successfully identified patients with unmet needs who would have been missed using a screening tool alone. Conversely, by applying interprofessional perspectives during rounds, we may have determined that formal PC consultation was unnecessary for patients who otherwise would have screened positive. This approach could be implemented in other inpatient areas to build awareness of PC needs and services, and improve others primary PC skills.
Our study has several limitations. First, to allow PC team members to participate and limit disruption to their other consultative work, enhanced discharge planning rounds were limited to twice per week. Therefore, patients who were admitted and discharged in between the interprofessional rounds never directly received the intervention. We included all patients in their original team assignments regardless of whether they were actually discussed (intention-to-treat), conservatively biasing our findings towards no result. Second, this study took place at a single academic medical center, on a non-resident hospitalist service. The results may not generalize to other services, or facilities without established PC consultation services with the capacity to accommodate additional consult requests for 1% of their non-cancer admissions. Because the two intervention teams by chance disproportionately admitted more cancer patients, we conservatively analyzed the results with a DID study design and controlled for differential effects on cancer versus non-cancer patients. Third, data on co-morbid conditions (which may affect decisions to consult PC) were limited to conditions found within our health system. We lacked a more precise way to differentially weight symptomatic and terminally-staged conditions expected to predispose patients to PC consultation. Therefore, we relied on the Charlson index, which is weighted by risk of dying. Last, we could not control for potential financial incentives related to increased consultation. Given the setting of an academic institution with salaried consultants and a high demand for PC, it is unlikely that they were incentivized to increase consultations. Thus, we presume all consultations provided were appropriate.
Future steps include an independent review of PC consult documentation to evaluate the services provided using PC Quality Network metrics.26 We also plan to compare this intervention with a validated screening tool, to determine which is more efficient at identifying patients requiring PC consultation. Assessing the durability of our findings after the completion of the study is another important direction for future study. Finally, we plan to study the adaptability of this approach to other inpatient areas (e.g surgery, oncology, medical ICU).
In conclusion, scheduled shared interprofessional rounds between hospitalists and PC specialists increased utilization and timeliness of PC consultations, particularly among non-cancer patients. Regular discussions between hospitalists and PC providers improved appropriate use of PC services and hospitalist PC skills.
Acknowledgements
Katherine Prenovost and Julia Mantey, for statistical consultation
Sarah Brand, for assistance with data cleaning and validation
This research was supported through Blue Cross Blue Shield of Michigan Foundation (BCBSM-15-PAF07639 01/2016-3/2017).
Footnotes
Conflicts of interest:
Rafina Khateeb, MD - none
Margaret R. Puelle, BS - none
Janice Firn, PhD, MSW - none
D’Anna Saul, MD - none
Robert Chang, MD - none
Lillian Min, MD, MSHS - none
Contributor Information
Rafina Khateeb, University of Michigan Medical School.
Margaret R. Puelle, University of Michigan Medical School.
Janice Firn, University of Michigan Medical School.
D’Anna Saul, University of Michigan Medical School.
Robert Chang, University of Michigan Medical School.
Lillian Min, University of Michigan Medical School; VA Ann Arbor Healthcare System, Geriatric Research Education and Clinical Center.
References
- 1.Institute of Medicine. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington DC: 2014. [Google Scholar]
- 2.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann C, Riechelmann R, Krzyzanowska M, Rodin G, Tannock I: Effectiveness of specialized palliative care: a systematic review. Jama. 2008;299(14):1698–1709. doi: 10.1001/jama.299.14.1698. [DOI] [PubMed] [Google Scholar]
- 4.Gade G, Venohr I, Conner D, et al. : Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180–190. doi: 10.1089/jpm.2007.0055. [DOI] [PubMed] [Google Scholar]
- 5.Ciemins EL, Blum L, Nunley M, Lasher A, Newman JM: The economic and clinical impact of an inpatient palliative care consultation service: a multifaceted approach. J Palliat Med. 2007;10(6):1347–1355. doi: 10.1089/jpm.2007.0065. [DOI] [PubMed] [Google Scholar]
- 6.May P, Garrido MM, Cassel JB, et al. : Prospective Cohort Study of Hospital Palliative Care Teams for Inpatients With Advanced Cancer: Earlier Consultation Is Associated With Larger Cost-Saving Effect. Journal of Clinical Oncology. 2015. doi: 10.1200/jco.2014.60.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison R, Penrod JD, Cassel J, et al. : Cost savings associated with us hospital palliative care consultation programs. Archives of Internal Medicine. 2008;168(16):1783–1790. doi: 10.1001/archinte.168.16.1783. [DOI] [PubMed] [Google Scholar]
- 8.Penrod JD, Deb P, Dellenbaugh C, et al. : Hospital-based palliative care consultation: effects on hospital cost. J Palliat Med. 2010;13(8):973–979. doi: 10.1089/jpm.2010.0038. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor NR, Moyer ME, Behta M, Casarett DJ: The Impact of Inpatient Palliative Care Consultations on 30-Day Hospital Readmissions. J Palliat Med. 2015;18(11):956–961. doi: 10.1089/jpm.2015.0138. [DOI] [PubMed] [Google Scholar]
- 10.Lefkowits C, Teuteberg W, Courtney-Brooks M, Sukumvanich P, Ruskin R, Kelley JL: Improvement in symptom burden within one day after palliative care consultation in a cohort of gynecologic oncology inpatients. Gynecol Oncol. 2015;136(3):424–428. doi: 10.1016/j.ygyno.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 11.Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A: Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med. 2015;18(2):134–142. doi: 10.1089/jpm.2014.0192. [DOI] [PubMed] [Google Scholar]
- 12.Weissman D, Meier D: Identifying Patients in Need of a Palliative Care Assessment in the Hospital Setting A Consensus Report from the Center to Advance Palliative Care. J Palliat Med. 2011; 14(1):. doi: 10:1089/jpm.2010.0347. [DOI] [PubMed] [Google Scholar]
- 13.Schenker Y, Arnold R: The Next Era of Palliative Care. Jama. 2015;314(15):1565–1566. doi: 10.1001/jama.2015.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes MT, Smith TJ: The growth of palliative care in the United States. Annu Rev Public Health. 2014;35:459–475. doi: 10.1146/annurev-publhealth-032013-182406. [DOI] [PubMed] [Google Scholar]
- 15.Humphreys J, Harman S: Late referral to palliative care consultation service: length of stay and in-hospital mortality outcomes. J Community Support Oncol. 2014;12(4):129–136. [DOI] [PubMed] [Google Scholar]
- 16.Lefkowits C, Binstock AB, Courtney-Brooks M, et al. : Predictors of palliative care consultation on an inpatient gynecologic oncology service: are we following ASCO recommendations? Gynecol Oncol. 2014;133(2):319–325. doi: 10.1016/j.ygyno.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Ferrell Betty R., Temel Jennifer S., Temin Sarah, Alesi Erin R., Balboni Tracy A., Basch Ethan M., Firn Janice I., Paice Judith A., Peppercorn Jeffrey M., Phillips Tanyanika, Stovall Ellen L., Zimmermann Camilla, and Smith Thomas J.: Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Onc. 2017; 35(1), 96–112. [DOI] [PubMed] [Google Scholar]
- 18.Finkelstein M, Goldstein NE, Horton JR, Eshak D, Lee EJ, Kohli-Seth R: Developing triggers for the surgical intensive care unit for palliative care integration. J Crit Care. 2016. Oct;35:7–11. doi: 10.1016/j.jcrc.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 19.Rocque GB, Barnett AE, Illig LC, et al. : Inpatient hospitalization of oncology patients: are we missing an opportunity for end-of-life care? J Oncol Pract. 2013;9(1):51–54. doi: 10.1200/jop.2012.000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL: Quality of End-of-Life Care Provided to Patients With Different Serious Illnesses. JAMA Intern Med. 2016;176(8):1095–1102. doi: 10.1001/jamainternmed.2016.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson JE, Curtis JR, Mulkerin C, Campbell M, Lustbader DR, Mosenthal AC, Puntillo K, Ray DE, Bassett R, Boss RD, Brasel KJ, Frontera JA, Hays RM, Weissman DE: Choosing and using screening criteria for palliative care consultation in the ICU: a report from the Improving Palliative Care in the ICU (IPAL-ICU) Advisory Board. Crit Care Med. 2013;41(10):2318–27. doi: 10.1097/CCM.0b013e31828cf12c. [DOI] [PubMed] [Google Scholar]
- 22.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 23.Elixhauser A, Steiner C, Harris DR, Coffey RM: Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 24.Karaca-Mandic P, Norton EC, Dowd B: Interaction Terms in Nonlinear Models. Health Services Research. 2012;47(1 Pt 1):255–274. doi: 10.1111/j.1475-6773.2011.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP. [Google Scholar]
- 26.Pantilat Steven Z, Marks Angela K, Bischoff Kara E, Bragg Ashley R, and O’Riordan David L: The Palliative Care Quality Network: Improving the Quality of Caring. J Palliat Med. 2017; 20(8): 862–868. 10.1089/jpm.2016.0514 [DOI] [PubMed] [Google Scholar]


