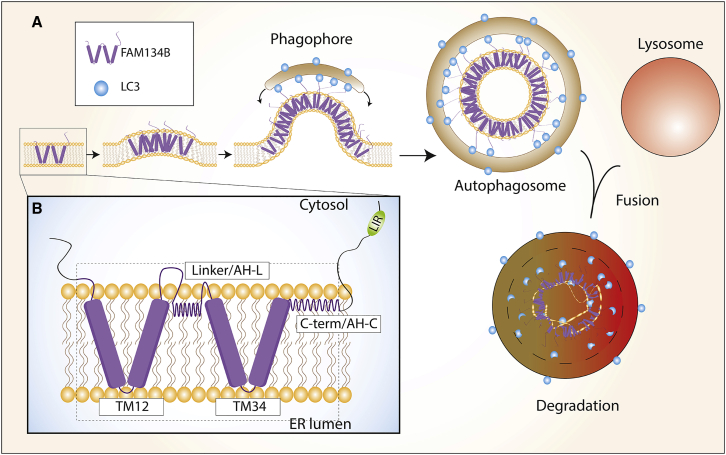
Figure 2.
Schematic representation of ER-phagy and reticulon-homology domains
(A) FAM134B, the best characterized RHD-containing ER-phagy receptor, can oligomerize, sense, and induce membrane curvature. High concentration of FAM134B leads to membrane budding, which is recognized by the autophagy machinery through direct binding between FAM134B and LC3/GABARAP proteins. This binding provides additional forces that lead to scission of these ER membrane buds and their subsequent incorporation into the forming autophagosome. The autophagosome will fuse with a lysosome, where the fragmented ER membrane and embedded RHD-containing proteins are degraded.
(B) RHDs assume a wedge-shaped form when inserted into the ER membrane. RHDs consist of two conserved transmembrane hairpins (TM1,2 and TM3,4) and two amphipathic helices (AH-L and AH-C). AH-L is part of a linker sequence, connecting TM1,2 and TM3,4, and AH-C is localized C-terminally of TM3,4. The transmembrane hairpins locally compress the lipid bilayer, while the amphipathic helices allow stretching of the cytosolic leaflet to induce strong local membrane deformations.