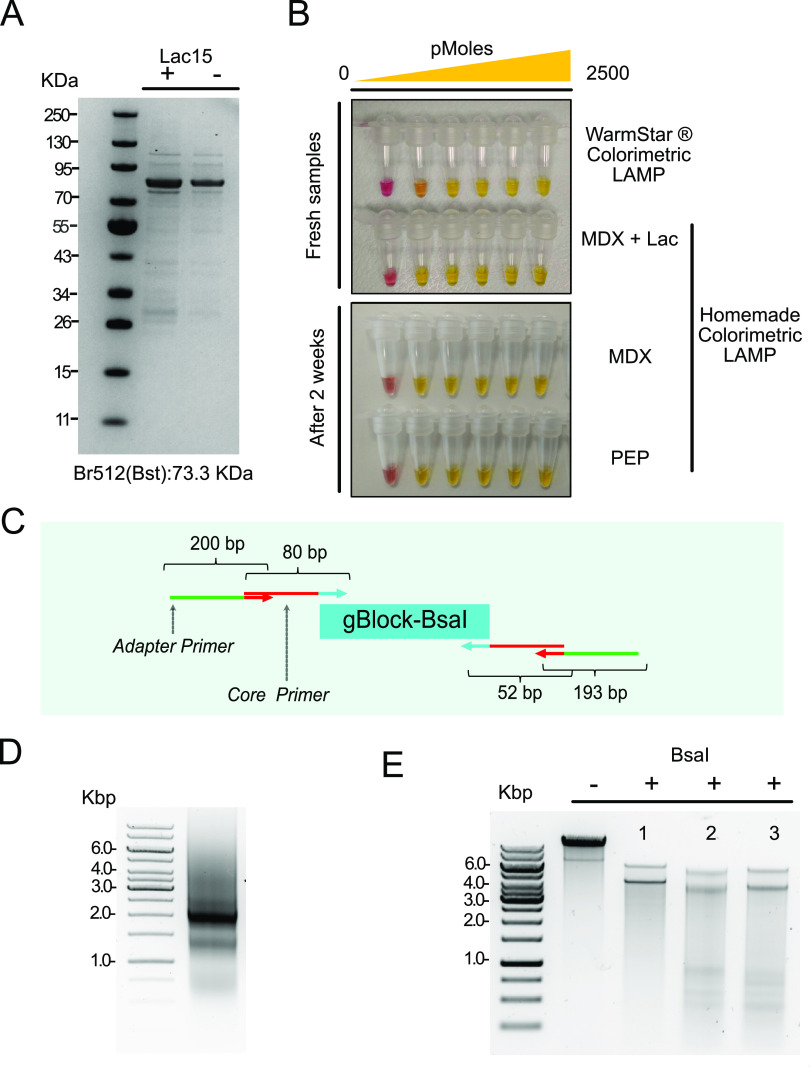
Figure 4.
Production of molecular biology reagents. (A) Purified Br512 Bst DNA polymerase visualized in a polyacrylamide gel stained with coomassie blue. (B) Colorimetric LAMP assay using the Br512 Bst DNA polymerase produced in vitro in both fresh conditions (top panel) and using rehydrated samples (bottom panel) after a 2 week storage at room temperature. Cell-free reactions based on PEP and MDX were prepared using the low-cost drying system and protected with sucrose (120 and 15 mM, respectively). A synthetic dsDNA fragment from actin B gene (Homo sapiens) was used as a target in the following amounts: 0, 0.025, 0.25, 2.5, 250, and 2500 pmoles. Primers used in this assay are described in Table S11. Negative reactions were pink-colored, and positive reactions changed to yellow. (C, D) A PCR product encoding the BsaI restriction endonuclease (2043 bp) was amplified using a single PCR with four oligonucleotides. An inner set of core primers provided a template for secondary amplification by longer oligonucleotides. The resulting product had extended terminal sequences that helped protect the coding region from exonuclease degradation. (E) Testing of BsaI by restriction endonuclease digestion of luxpGEX plasmid. Digestion was performed using BsaI produced by cell-free technology. Plasmid DNA samples were treated with (1) FastDigest Eco31I (Thermo Scientific, FD0293) (Isoschizomer: BsaI), (2) BsaI in cell extract, and (3) BsaI in cell extract: 100% glycerol (1:1). Expected size of bands after digestion: 6440 and 4433 bp.