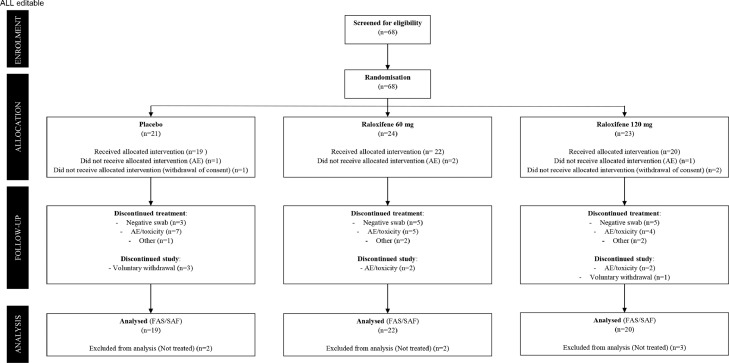
Figure 1.
Trial profile.
All consented patients were eligible to be enrolled in the trial. The Safety (SAF) and the Full Analysis Set (FAS) populations consist of all randomized patients who received at least one dose of the investigational product. The SAF population was analyzed according to the actual treatment received, while the FAS population according to ITT principle, i.e. by treatment allocation.
AE: Adverse Event.