Figure 1.
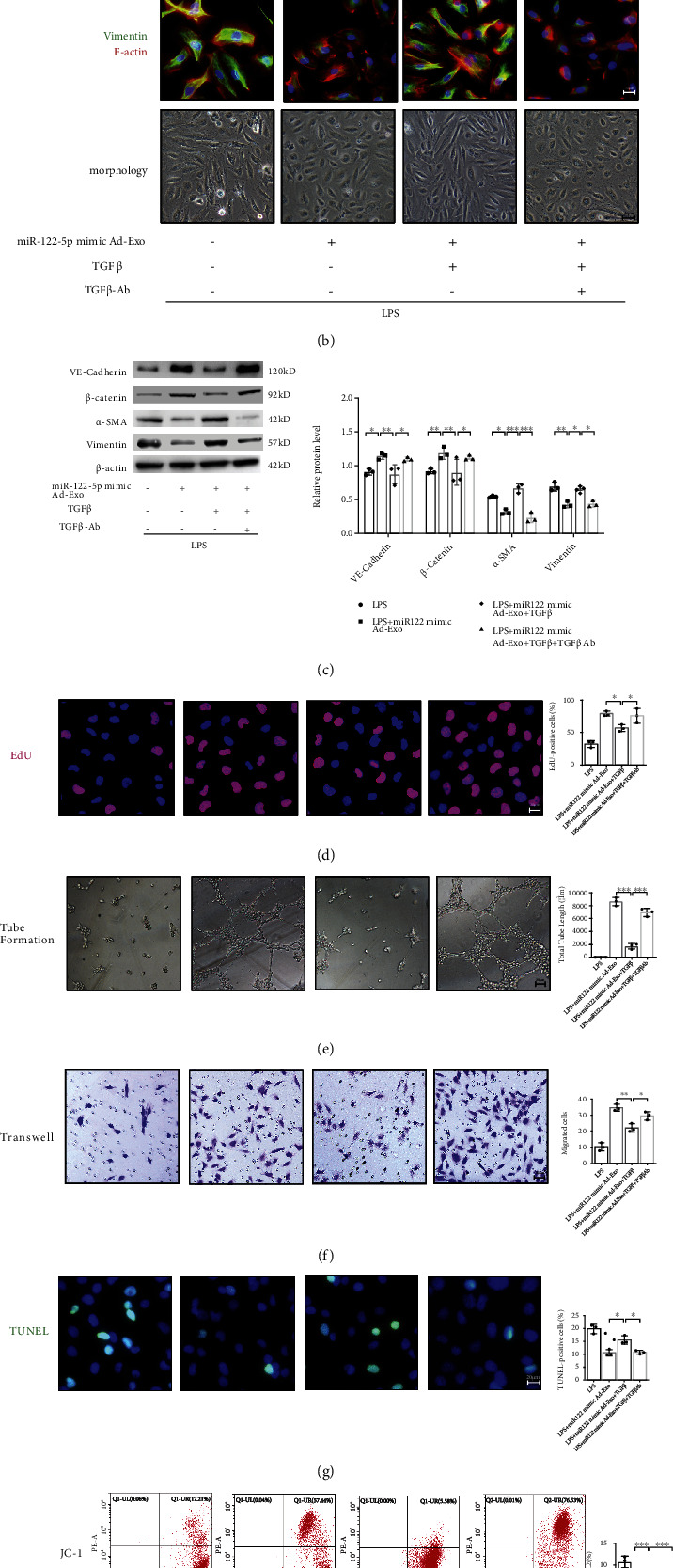
Adipocyte-derived exosomal miR-122-5p reinforces the pulmonary endothelial barrier by inhibiting EndMT and oxidative stress through down-regulating the TGF-β1/TGF-βR1/Smad2 pathway in HPMECs. (a) Dual-Luciferase reporter assay showed that human TGF-β1 is a direct target of miR-122-5p through the binding site in its 5′-UTR. The firefly luciferase activity of the reporter containing the 5′UTR-WT of human TGF-β1 was decreased to 62.61% by miR-122-5p, which was blocked by the reporter containing the 5′-UTR -MUT of human TGF-β1 (C787G, C797T, C807T). (b) Immunofluorescence staining, phalloidin F-actin staining, and cell morphology of HPMECs showed that the inhibitory effects of exosomal miR-122-5p on EndMT in LPS-insulted HPMECs were blocked by TGF-β1 treatment, but TGFβ1-Ab overturned these effects. (c) Western blot analysis of the protein expression of VE-cadherin, β-catenin, α-SMA, and Vimentin in HPMECs. Relative abundances of protein bands were normalized to β-actin, as shown in the bar graphs. EdU staining (d), tube formation (e), transwell (f), TUNEL staining (g), and JC-1 staining (h) showed that the angiogenic and antioxidant capacity of exosomal miR-122-5p was abrogated by TGF-β1 treatment (5 ng/mL for 6 hours), but TGF-β1-Ab (1.25 μg/ml for 6 hours) partially restored these capacities. (i) Western blot analysis of the protein expression of TGF-β1, and the phosphorylation of TGF-βR1 (p-TGF-βR1) and Smad2 (p-Smad2) in HPMECs. Relative abundances of protein bands were normalized to β-actin as shown in the bar graphs. Relative phosphorylation levels of protein are expressed normalized to the corresponding total protein. Representative images are shown from three replicated independent experiments. n = 6 per group (a). n = 3 per group for other groups. Data are presented as mean ± S.D. Significant differences are shown by ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
