Figure 7.
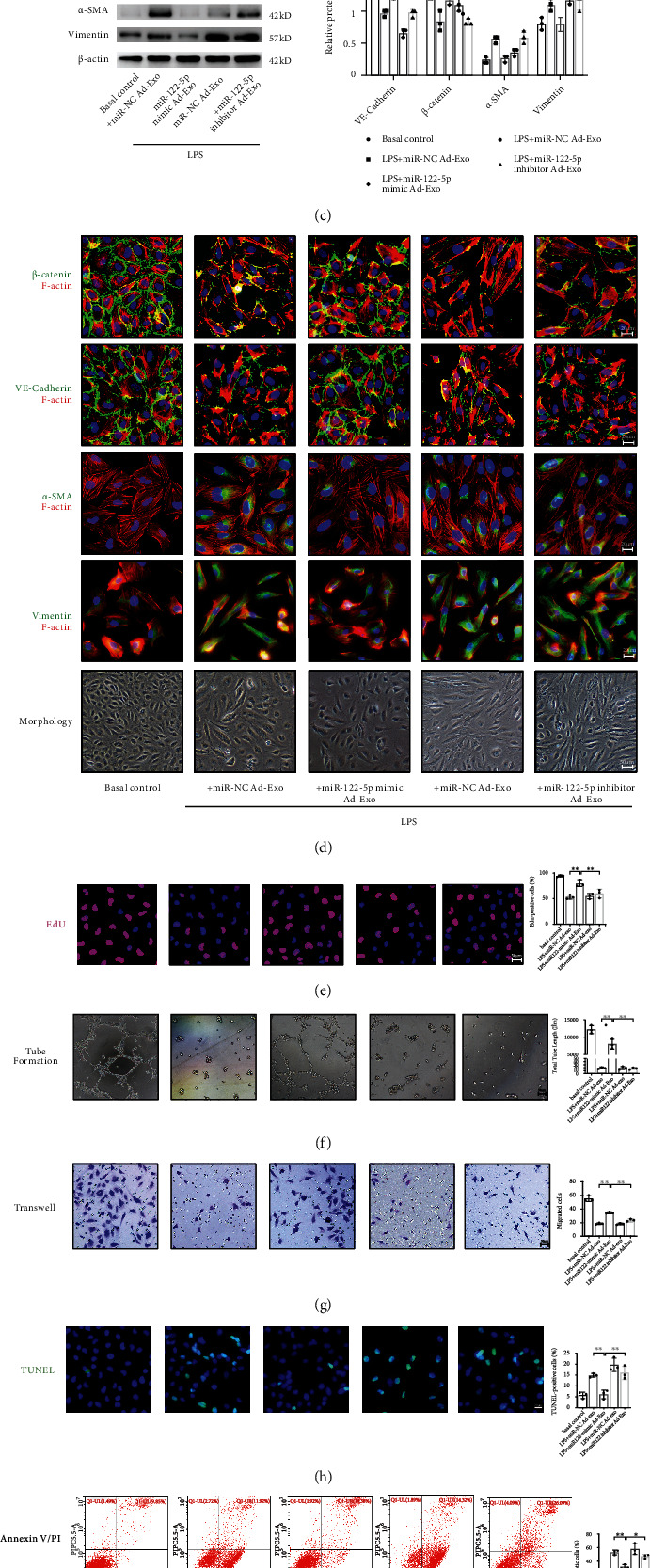
Adipocyte-derived exosomal miRNA-122-5p reinforces the pulmonary endothelial barrier by inhibiting EndMT and oxidative stress in HPMECs. (a) Adipocyte-derived exosomes were labeled with PKH-26 dye, and the appearance of red fluorescence in CD31-marked HPMECs confirmed that adipocyte-derived exosomes were taken up by HPMECs. Scale bar = 20 μm. (b) Adipocytes transfected with 5-FAM-labeled miR-122-5p mimics were cocultured with HPMECs in Transwell plates. 5-FAM-labeled miR-122-5p mimics were delivered from adipocyte to HPMECs, as evidenced by the appearance of green fluorescence concomitant with a 5.19-fold increase in miR-122-5p abundance in HPMECs. (c) Western blot analysis of the protein expression of VE-cadherin, β-catenin, α-SMA, and Vimentin in HPMECs. Relative abundances of protein bands were normalized to β-actin, as shown in the bar graphs. (d) Immunofluorescence staining, phalloidin F-actin staining, and cell morphology of HPMECs showed a transition from the flattened endothelial phenotype to spindled mesenchymal-like phenotype, along with a decrease in VE-cadherin and β-catenin, and a slight increase in α-SMA and Vimentin under LPS insult conditions (100 ng/mL for 72 hours), which were attenuated by adipocyte-derived exosomes pretreated with miR-122-5p mimics. Scale bar = 20 μm. EdU staining (e), tube formation (f), transwell (g), TUNEL staining (h), Annexin V/PI staining (i), and JC-1 staining (j) showed that adipocyte-derived exosomes that were transfected with miR-122-5p mimics increased EdU staining positive HPMECs, tube length, and cell mitigation, whereas attenuated apoptosis and oxidative stress under LPS stimulation. These effects were reversed by adipocyte-derived exosomes that were transfected with miR-122-5p inhibitor. Representative images are shown from three replicated independent experiments. n = 3 per group. Data are presented as mean ± S.D. Significant differences are shown by ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
