Abstract
Neurofilament light chain (NfL) is a novel biomarker reflecting neuroaxonal damage and associates with brain atrophy, and glial fibrillary acidic protein (GFAP) is a marker of astrocytic activation, associated with several neurodegenerative diseases. Since obesity is associated with increased risk for several neurodegenerative disorders, we hypothesized that circulating NfL and GFAP levels could reflect neuronal damage in obese patients. 28 morbidly obese and 18 lean subjects were studied with voxel based morphometry (VBM) MRI to assess gray and white matter densities. Serum NfL and GFAP levels were determined with single-molecule array. Obese subjects were re-studied 6 months after bariatric surgery. Morbidly obese subjects had lower absolute concentrations of circulating NfL and GFAP compared to lean individuals. Following bariatric surgery-induced weight loss, both these levels increased. Both at baseline and after weight loss, circulating NfL and GFAP values correlated inversely with eGFR. Cross-sectionally, circulating NfL levels correlated inversely with gray matter (GM) density, and this association remained significant also when accounting for age and total eGFR. GFAP values did not correlate with GM density. Our data suggest that when determining circulating NfL and GFAP levels, eGFR should also be measured since renal function can affect these measurements. Despite the potential confounding effect of renal function on NfL measurement, NfL correlated inversely with gray matter density in this group of subjects with no identified neurological disorders, suggesting that circulating NfL level may be a feasible biomarker of cerebral function even in apparently neurologically healthy subjects.
Subject terms: Neuroscience, Medical research, Neurology
Introduction
Obesity and obesity-related impairments in glucose homeostasis present as risk factors for several neurodegenerative disorders, including Alzheimer’s disease (AD)1,2. With the ever increasing obesity pandemic3,4, the risk for increasing prevalence of neurodegenerative disorders is imminent.
Magnetic resonance imaging (MRI) is the current standard to quantitate brain atrophy as a macroscopic reflection of the neuroaxonal damage due to disease or aging. Obesity has also been shown to associate with decreased brain volume5, and importantly, these structural changes in obesity have been suggested to reflect early stages of neuronal damage, possibly leading to irreversible neurodegeneration in obesity6. However, MRI is a relatively expensive neuroimaging tool and not easily accessible to a large number of patients. Less expensive circulating biomarkers would be essential as a screening tool for brain atrophy.
A new potential biomarker of neurodegeneration is neurofilament light chain (NfL), which is one of the scaffold proteins specific for neuronal cytoskeleton7. Upon neuro-axonal injury, NfL is released into extracellular space8, and elevated NfL levels in the cerebrospinal fluid and peripheral blood have been shown to indicate axonal injury in several neurologic diseases9,10. Glial fibrillary acidic protein (GFAP) is an intracellular protein of the astrocytic cytoskeleton, and increases in central nervous system (CNS) GFAP and circulating GFAP levels have been described in the context of traumatic brain injury (TBI)11, in the context of neurodegeneration in AD12 as well as in chronic neuroinflammatory conditions such as multiple sclerosis (MS)13.
We have previously shown that obesity is linked to decreased brain density, and that weight loss following bariatric surgery leads to global increase in white matter (WM), and increase in gray matter (GM) density in occipital and inferior temporal regions14. In this study, we further evaluated the effect of obesity and weight loss following bariatric surgery on the circulating levels of NfL and GFAP, on the same subjects reported by Tuulari et al.14. Moreover, we assessed whether circulating NfL and GFAP correlate with brain density.
Materials and methods
Participants and study design
The initial dataset comprised of 28 morbidly obese and 19 lean controls. All study participants were free of neurological disease (e.g., large vessel stroke, seizure disorder, Parkinson’s or Alzheimer’s disease, clinically significant traumatic brain injury, multiple sclerosis, or previous brain infection/meningitis); and none of them had any major psychiatric illness (e.g., schizophrenia, bipolar disorder), nor reported substance abuse. Subjects were also eligible to undergo magnetic resonance imaging. In one lean subject, the NfL level was clearly in the abnormal range (NfL value 37.8 (ng/L)). After checking her medical records, we identified that the subject had undergone surgery for a large lumbar disc prolapse causing stenosis 9 months before her participation in the study. As increases in circulating NfL levels may be associated with herniated and spondolytic intervertebral discs15, we excluded this subject from further analysis, leaving 28 morbidly obese and 18 lean controls in the dataset. Patients with obesity were studied before and six months after bariatric surgery. Lean subjects were studied once. Inclusion and exclusion criteria and the surgical techniques have been previously described in detail16. Altogether, 8 subjects from the morbidly obese group had pre-diabetic conditions (impaired fasting glucose (IFG) or impaired glucose tolerance (IGT)) and 11 had type 2 diabetes mellitus (T2D) as defined by ADA criteria17. All lean subjects had normal glucose tolerance (NGT). The study protocol was approved by the Ethics Committee of the Hospital District of Southwest Finland, all research was performed in accordance with relevant guidelines/regulations and all subjects gave their written informed consent before participating in the study (NCT00793143 and NCT01373892).
Neurofilament and GFAP measurement
The analyses of serum NfL and GFAP were performed by the Biomarkers for Neurodegenerative Disorders research group in University of Eastern Finland (Kuopio, Finland; https://www3.uef.fi/fi/web/neuro/biomarkers) using the single-molecule array (SIMOA) platform (Quanterix Corporation, Billerica, MA, USA) (“Advantage” and “Discovery” kits for NfL and GFAP, respectively). In 8/46 subjects the GFAP assessment failed due to high replicate variance (coefficient of variation (CV) > 20%), and thus, GFAP data were available for evaluation in 38 subjects. Mean CV of the sample replicates was 5.2% for NfL and 6.9% for GFAP. Variance of control material was within acceptable limits based on internal quality control protocol (± 3SD).
Analytical methods
Plasma glucose was measured in the laboratory of Turku PET Centre in duplicate using the glucose oxidase technique (Analox GM7 or GM9, Analox Instruments Ltd., London, UK). Glycosylated hemoglobin (HbA1c) was measured with ion-exchange high performance liquid chromatography (Variant II Haemoglobin A1c, Bio-Rad Laboratories, CA, USA). Plasma insulin was determined by time-resolved immunofluorometric assay (AutoDELFIA, Perkin Elmer Inc, Wallac Oy, Turku, Finland). Serum FFA were measured with a photometric enzymatic assay (Wako Chemicals GmbH, Neuss, Germany) on Modular P800 automatic analyzer (Roche Diagnostics, Mannheim, Germany). Serum high-sensitivity C-reactive protein (hs-CRP) was analysed with the sandwich immunoassay method using an Innotrac Aio1 immunoanalyzer (Innotrac Diagnostics, Turku Finland). Serum adipokines were analysed in duplicate by using Milliplex Human Serum Adipokine (Panel A) kit [cat.no HADK1-61K-A] (MilliporeSigma, Burlington, MA, USA) containing interleukin-6 (IL-6), interleukin-8 (IL-8), tumor necrosis factor alpha (TNFα), and leptin.
MRI studies
MR imaging was performed with Philips Gyroscan Intera 1.5 T CV Nova Dual scanner at Turku PET Centre. 3D anatomical images with isotropic 1 mm3 resolution were acquired using a T1-weighted sequence (TR 25 ms, TE 4.6 ms, flip angle 30, scan time 376 s, image dimensions (X,Y,Z): 256 × 256 × 330, FFE SENSE protocol).
Voxel-based morphometry (VBM)
Prior to analysis, the image quality was checked visually, and the origo of each T1 image was set to anterior commissure. Structural images were spatially normalized and segmented using the MAGIA pipeline (https://aivo.utu.fi/magia/) which utilizes Matlab (The MathWorks Inc., Natick, MA) and the Statistical Parametric Mapping toolbox (SPM 12) (http://www.fil.ion.ucl.ac.uk/spm/). Additionally, the DARTEL algorithm (in SPM12)18 was applied to achieve non-linear correction for GM and WM that takes into account the changes in brain volume and minimizes possible biases via creation of multiple templates. Finally, the GM and WM images, at this stage called GM density and WM density images, were smoothed using a Gaussian kernel of 8 mm full width at half maximum (FWHM).
Total blood volume equations
Total blood volume was estimated according to the Nadler’s formula19. Since this formula has been criticized for overestimating total blood volume in extreme BMI range and we had some subjects with morbid obesity, we also calculated total blood volume using the Lemmens formula20. These measurements of estimated total blood volume correlated positively and strongly to one another (r = 0.99, p < 0.0001).
Estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation21.
Body surface area (BSA) was estimated as previously described by Du Bois&Du Bois22.
Statistical analysis
Data are presented as mean ± SD (or median [IQR] for non-normally distributed variables). Whole-brain statistical analysis was performed with SPM12 running on Matlab. Linear regressions were performed in SPM to evaluate correlations between VBM and single regressors (NFL, GFAP) while controlling for confounding factors (in the SPM contrast, the controlling variables were set to a value of 0). The statistical threshold in SPM analysis was set at a cluster level and corrected with false discovery rate (FDR) with p < 0.05. Further statistical analyses were done using JMP version 13.0 (SAS Institute, Cary, NC, USA). A p value < 0.05 was considered statistically significant.
Results
Baseline
Our population consisted predominantly of women (91%). As expected, obese subjects were more insulin resistant compared to the lean individuals and had higher circulating inflammatory marker (CRP) and higher leptin levels. Even though obese subjects had non-significantly lower total cholesterol values from the lean controls, they had significantly lower HDL cholesterol values. There were no differences in eGFR (ml/1.73 m2/min) between obese and lean individuals, but eGFR expressed in ml/min accounting for each subject’s BSA was significantly higher in patients with obesity. There was no difference between the two groups in terms of blood pressure and age. The baseline characteristics of the study participants are reported in Table 1.
Table 1.
Anthropometric and biochemical characteristics of the study participants.
| Controls | Obese | p value | ||
|---|---|---|---|---|
| Pre | Post | |||
| M/W | 2/16 | 2/26 | – | 0.7 |
| NGT/IFG&IGT/T2D | 19/0/0 | 9/8/11 | 22/2/4 | < 0.0001 |
| Age (years) | 45 ± 10 | 45 ± 10 | – | 0.2 |
| BMI (kg m−2) | 23.4 [3.5] | 41.2 [5.7] | 29.6 [5.7]*,# | < 0.0001 |
| HbA1c (%) | 5.6 [0.5] | 5.9 [0.6] | 5.6 [0.5]* | 0.6 |
| Systolic BP (mmHg) | 131 ± 13 | 137 ± 16 | 128 ± 15* | 0.4 |
| Diastolic BP (mmHg) | 82 ± 9 | 86 ± 10 | 80 ± 9* | 0.1 |
| Total cholesterol (mmol/L) | 4.6 ± 0.9 | 4.2 ± 0.8 | 4.1 ± 0.7 | 0.2 |
| LDL cholesterol (mmol/L) | 2.4 ± 0.7 | 2.4 ± 0.7 | 2.2 ± 0.7 | 0.9 |
| HDL cholesterol (mmol/L) | 1.8 ± 0.4 | 1.2 ± 0.3 | 1.4 ± 0.3* | < 0.0001 |
| Triglycerides (mmol/L) | 0.7 ± 0.3 | 1.3 ± 0.5 | 1.1 ± 0.5* | < 0.0001 |
| Smoking (yes/no) | 0/19 | 6/22 | 2/22* | 0.03 |
| Menopause (yes/no) | 10/9 | 12/16 | 12/16 | 0.5 |
| CRP (mg/L) | 0.6 [1.0] | 3.1 [4.0] | 1.1 [1.4] | < 0.0001 |
| IL-6 (ng/L) | 1.8 [2.0] | 2.5 [1.3] | 1.9 [0.3] | 0.2 |
| IL-8 (ng/L) | 4.3 [3.1] | 5.1 [3.7] | 8.1 [5.3] | 0.1 |
| Leptin (μg/L) | 5.6 [9.6] | 48.8 [25.9] | 25.5 [15.5]*,# | < 0.0001 |
| TNFα (ng/L) | 4.0 [3.9] | 4.7 [3.1] | 7.5 [2.9] | 0.2 |
| HOMA-IR | 1.3 [1.8] | 3.2 [4.1] | 1.5 [1.2] | < 0.0001 |
| Total intracranial volume (L) | 1.5 [0.2] | 1.4 [0.1] | 1.4 [0.1] | 0.04 |
| Serum creatinine (μmol/L) | 61 ± 11 | 61 ± 7 | 62 ± 11 | 0.7 |
| eGFR (ml/1.73 m2/min) | 102 ± 10 | 104 ± 12 | 103 ± 15 | 0.9 |
| Total eGFR (ml/min) | 103 ± 10 | 131 ± 21 | 117 ± 20* | < 0.0001 |
| Neurofilament (ng/L) | 10.7 [5.0] | 6.7 [4.0] | 9.5 [5.1]* | 0.002 |
| GFAP (ng/L) | 208 [111] | 135 [72] | 169 [112]* | 0.01 |
Entries are mean ± SD, or median [interquartile range], as appropriate. p value for the comparison between obese pre and lean individuals; *p < 0.05 for obese before and after bariatric surgery; #p < 0.05 for the comparison obese post vs. lean individuals. BMI body mass index, NGT normal glucose tolerance, IFG impaired fasting glucose, IGT impaired glucose tolerance, T2D type 2 diabetes, BP blood pressure, CRP C-reactive protein, HOMA-IR homeostatic model assessment of insulin resistance, eGFR estimated glomerular filtration rate, GFAP glial fibrillary acidic protein.
In the pooled data, NfL correlated positively with age (r = 0.64, p < 0.0001), and negatively with BMI (r = − 0.32, p = 0.03) among the 46 study participants (Fig. 1). In the 38 subjects who also had circulating GFAP values measured, there was a positive correlation between NfL and GFAP (r = 0.49, p = 0.002) (Fig. 1). In the pooled data, NfL and GFAP concentrations correlated inversely with estimated glomerular filtration rate expressed per 1.73 m2 of body surface area (BSA) (r = − 0.45, p = 0.002, r = − 0.54, p = 0.0005, respectively). This correlation became stronger when estimated glomerular filtration rate was expressed in ml/min (r = − 0.50, p = 0.0003 and r = − 0.58, p = 0.0001, for NfL and GFAP respectively) (Fig. 2). In a multivariate analysis, age (standardized β = 0.62, t ratio 5.0, p < 0.0001) and interaction between age and eGFR (β = − 0.24, t ratio − 2.2, p = 0.033) were the best predictors for NfL concentrations, and BMI (β = − 0.30, t ratio − 2.0, p = 0.055) had a borderline association, whereas eGFR did not show meaningful independent contribution (β = − 0.15, t ratio − 0.8, p = 0.407). Total blood volume was not included in this multivariate model due to its high correlation with BMI (r = 0.84) and eGFR (r = 0.78).
Figure 1.
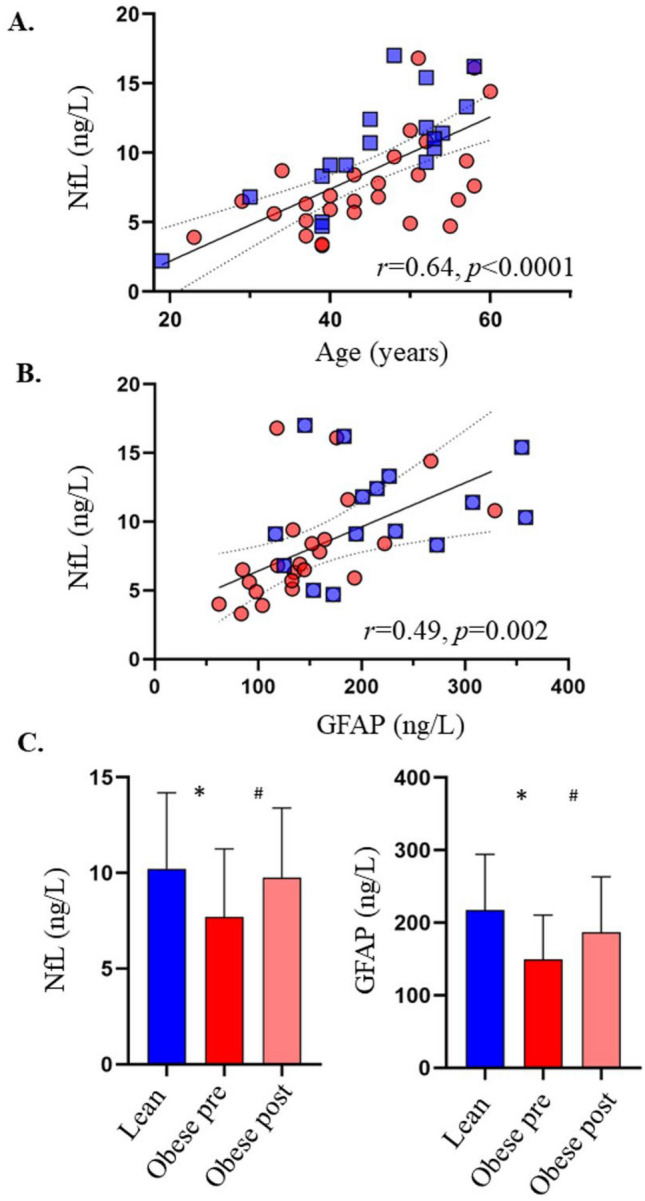
Plasma NfL levels correlated positively with age (A), and with GFAP concentrations (B). Plasma NfL and GFAP were higher in the lean as compared to the obese individuals, and increased 6-months post bariatric surgery (C). Red circles: subjects with obesity; blue squares: lean subjects. Light pink: obese subject post bariatric surgery. GFAP glial fibrillary acidic protein, NfL Neurofilament light chain.
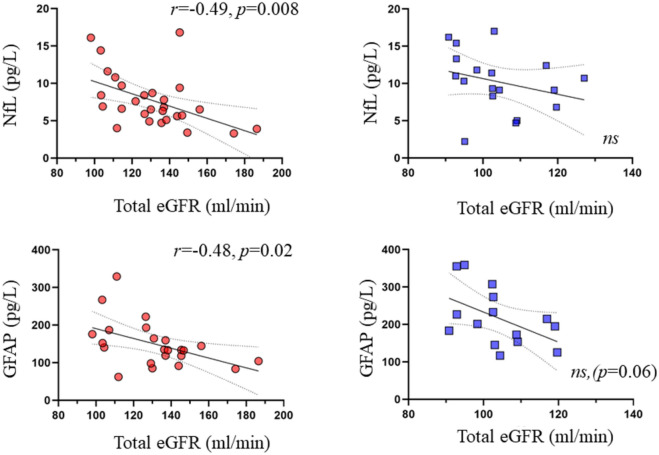
Figure 2.
The correlation between NfL and GFAP with total eGFR was driven by the obese subjects. Red circles: subjects with obesity; blue squares: lean subjects.
Regarding GFAP, in univariate analysis age (r = 0.52, p = 0.0009), BMI (r = − 0.41, p = 0.001), eGFR (r = − 0.58, p = 0.0001), and total blood volume (r = − 0.32, p = 0.052) were all significantly related to GFAP. In multivariate analysis, only age (β = 0.36, t ratio − 2.2, p = 0.036) was significant predictor for GFAP, whereas interaction between age and eGFR (β = − 0.26, t ratio − 1.9, p = 0.068) and eGFR (β = − 0.36, t ratio − 1.6, p = 0.117) had borderline associations, and BMI (β = − 0.17, t ratio − 0.9, p = 0.369) did not show meaningful independent contribution.
Following bariatric surgery
Patients undergoing bariatric surgery achieved significant weight loss with an average weight loss of approximately 10 units of BMI at six months after surgery. Insulin sensitivity, glucose tolerance status, and markers of systemic inflammation were also improved (Table 1). Circulating NfL and GFAP values were increased, and total eGFR decreased after surgery, and at the postoperative assessment, there was no difference with the plasma NfL and GFAP values of the lean subjects, and they still correlated positively with each other (r = 0.50, p = 0.01) (Table 1, Figs. 1, 3). Furthermore, circulating NfL and GFAP values were still negatively correlating with estimated glomerular filtration rate (Fig. 3).
Figure 3.
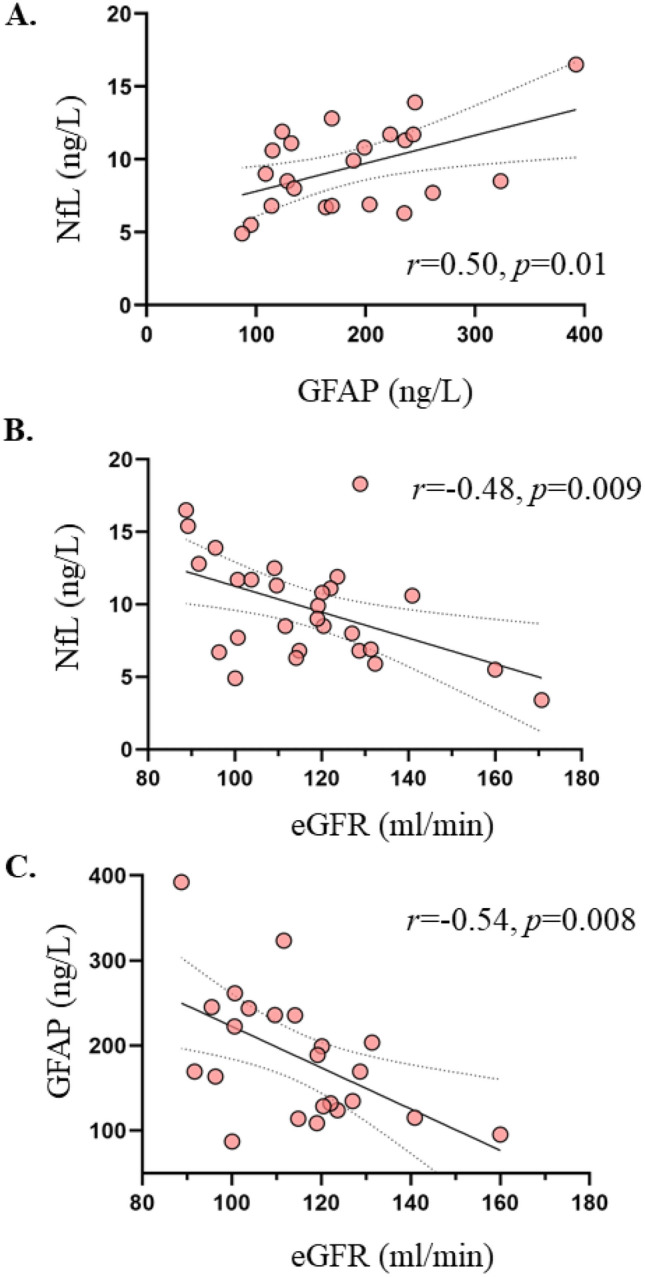
6-months after bariatric surgery circulating NfL and GFAP levels were directly related to each other (A), and correlated inversely with total eGFR (B, C). GFAP glial fibrillary acidic protein, NfL neurofilament light chain, eGFR estimated glomerular filtration rate.
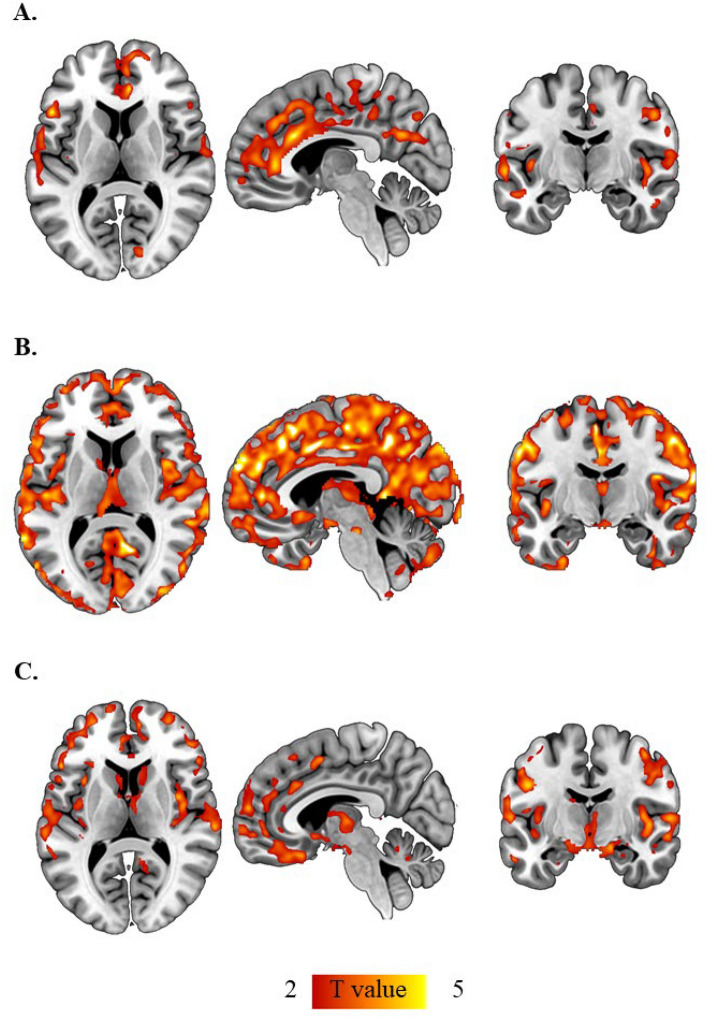
NfL and VBM
In the whole dataset, circulating NfL levels correlated inversely with GM density in hippocampi, cingulate gyrus and in wide-spread and partially scattered areas in frontal, parietal and occipital lobes. When dividing the population into obese and lean individuals, lean subjects showed widespread correlations between cortical GM and medial thalamic densities and NfL, whereas in the obese subjects the correlation was restricted mainly to the frontotemporal cortices, head of caudate, and hippocampi (Fig. 4). In both groups, the correlations between GM density and NfL levels remained significant after accounting for age and total blood volume, or after accounting for age and glomerular filtration rate (Supplementary Fig. 1A,B). No correlation was found between white matter density and circulating NfL levels. GFAP did not correlate with gray or white matter densities in the whole dataset, or when dividing the population in the two subgroups.
Figure 4.
Plasma Neurofilament light chain correlated inversely with gray matter density in the whole dataset (A). This correlation was more widespread in the lean (B), compared to the obese (C) individuals. Statistical parametric mapping results (p < 0.05, false discovery rate (FDR) corrected; extent threshold k: 9937 (A), extent threshold k: 1120 (B); Extent threshold k: 7614 (C), X: 5, Y: − 10, Z: 9.5). Images were created using Mango (Multi-image Analysis GUI) software, version 4.1 (http://rii.uthscsa.edu/mango/).
At 6 months after bariatric surgery, circulating NfL levels were still negatively associated with GM density (Supplementary Fig. 1C).
Discussion
In a sample of young and middle-aged individuals, across a wide range of BMI and insulin sensitivity with no clinical diagnosis of neurologic disorders, circulating plasma NfL levels correlated inversely with gray matter density in hippocampi, cingulate gyrus, and in frontal, parietal, and occipital lobe regions. In contrast to our hypothesis plasma NfL and GFAP concentrations were lower in patients with obesity compared to lean subjects, but at six months after bariatric surgery with significant weight loss, NfL and GFAP levels were increased to the same level as in lean controls. While circulating NfL and GFAP concentrations were directly related both at pre- and post-surgery, we found a significant inverse correlation between both biomarkers and estimated glomerular filtration rate both at baseline and following bariatric surgery.
NfL has been proposed as a useful biomarker for several neurologic diseases since increased NfL concentrations in the cerebrospinal fluid or blood indicate axonal injury9,10. Since obesity is linked to decreased brain density14, and it represents a risk factor for neurodegeneration23, our hypothesis was that morbidly obese subjects would have increased circulating NfL values, and that following weight loss NfL values would have been decreased. Similarly, GFAP is expressed in activated astrocytes24, and is a marker for astrogliosis in the CNS24, and thus, we would have expected higher circulating GFAP levels in the obese25–28. Contrary to our hypotheses, we found higher circulating levels of both these two biomarkers in the lean subjects compared to the obese individuals.
Indeed, in a recent large study, Manouchehrinia and colleagues have shown that BMI and blood volume correlate negatively with circulating plasma NfL levels, whereas no such association was found between CSF NfL levels and BMI29. Importantly, one key feature in obesity is glomerular hyperfiltration30 which in the long run constitutes the basis for obesity-induced chronic renal failure31. Our data showed that in patients with obesity, both biomarkers correlated inversely with eGFR both at baseline and after bariatric surgery, suggesting that the degree of renal function may affect their circulating levels. This finding is in agreement with the recent report by Akamine et al., where it was also shown that circulating plasma NfL levels correlated positively with serum creatinine and negatively with estimated glomerular filtration rate (eGFR) in healthy controls and patients with T2D32. Further, in the multivariate regression analyses, age and eGFR interaction term was a significant predictor for NfL and had a similar trend for GFAP suggesting that the effect of age on circulating NfL and GFAP concentrations are partially mediated by eGFR further highlighting the finding by Akamine et al.
Obesity is a risk factor for microalbuminuria and proteinuria33. Pathophysiological mechanisms include alteration of the tubulo-glomerular feedback, endothelial dysfunction linked to chronic microinflammation33, and reduced reabsorption in the proximal tubules34. The molecular weights of NfL (61.5 kDa35) and GFAP (50 kDa36) are similar to that of albumin (66.5 kDa), and thus, obesity could also lead to relatively higher loss of these proteins in urine, explaining the decreased circulating levels of NfL and GFAP in the obese.
In further support of this, the association between NfL (or GFAP) and eGFR was driven by the subjects with obesity, whereas no such correlation was found in the lean individuals. Unfortunately, urine samples were not collected in the current dataset, and thus, future research is warranted to establish whether NfL and GFAP are cleared by the kidneys, and whether their excretion correlates with the level of albuminuria. More specifically, a study assessing contemporaneously CSF, plasma, and urine concentrations of NfL and GFAP would be most informative. This could also have implications on attempts to establish diagnostic reference values as well as on interpreting longitudinal changes and treatment responses in neurologic diseases.
Despite a significant interaction with glomerular filtration rate, we found an inverse correlation between NfL levels and gray matter density. This inverse correlation may reflect an association between neuroaxonal damage and decreased neuronal density in obesity. However, even more wide-spread correlations were observed among healthy controls, which warrants further studies. Since our dataset comprised of apparently neurologically healthy individuals who were discordant only in terms of BMI, we hypothesize that the stronger correlations of GM density with NfL levels in lean subjects is suggestive of the more accurate measurement of NfL in these subjects. Circulating NfL values may be less representative in the obese because of higher filtration and excretion of the protein in subjects with obesity, thus masking the correlation with GM density to some extent. In line with our findings, another recent study has also demonstrated that higher circulating NfL levels associate with lower total brain volumes and higher age37. The association between decreased brain density and higher circulating NfL in our study may also represent a preceding stage before the development of more gross atrophy, which may be driven by normal aging as well as subclinical comorbidities37.
On the contrary, we did not find any association between circulating GFAP levels and GM density. However, one salient finding of the present study is the close correlation between NfL and GFAP concentrations both in the pooled data in obese and lean individuals, and in obese subjects six months following bariatric surgery induced weight loss. This finding is in accordance with previous studies showing the close correlation of the two circulating proteins in patients with MS38 and in healthy controls39,40. What is of particular interest in the present study, is that this close correlation was also found in neurologically healthy subjects. From a pathophysiologic standpoint, in CNS injury astrocytes get activated and proliferate expressing the GFAP in an attempt to limit the insulting process41. However, astrocytes may also secrete proinflammatory mediators and thus eventually contribute to the CNS damage42. Interestingly, diet-induced obesity and high-fat diet may be associated with hypothalamic inflammation and concomitant astrogliosis and GFAP overexpression43, but in order to detect subtle morphometric changes in hypothalamus and their associations with circulating GFAP levels, larger sample sizes than in our current study may be needed.
There are some important limitations in this study. First, our sample comprised mainly of women, and thus, extrapolation of our findings to men should be done with caution. Even though our studied population comprised of apparently neurologically healthy individuals, cognitive function was not formally evaluated. Thus, in future research it would be interesting to study whether circulating NfL levels correlate with cognitive function using standardized and validated assessment methods in subjects with morbid obesity.
In conclusion, our data suggest that decreased circulating NfL and GFAP levels found in patients with obesity may be based on their enhanced glomerular filtration rate. However, circulating NfL correlated inversely with gray matter densities in this dataset comprised of apparently neurologically healthy subjects, suggesting that this circulating biomarker may be useful even in detecting associations with subtle alterations in brain density. Based on our data, we suggest considering other possible confounders, such as BMI and GFR, when determining plasma NfL and GFAP.
Supplementary Information
Acknowledgements
The authors thank the staff of the Turku PET Centre for performing the PET imaging. No potential conflicts of interest relevant to this article were reported.
Abbreviations
- AD
Alzheimer’s disease
- BMI
Body mass index
- eGFR
Estimated glomerular filtration rate
- GFAP
Glial fibrillary acidic protein
- GM
Gray matter
- IFG
Impaired fasting glucose
- IGT
Impaired glucose tolerance
- MRI
Magnetic resonance imaging
- NFL
Neurofilament light chain
- NGT
Normal glucose tolerance
- Simoa
Single-molecule array
- SPM
Statistical parametric mapping
- T2D
Type 2 diabetes
- VBM
Voxel-based morphometry
- WM
White matter
Author contributions
Design: E.R., P.N.; conduct/data collection: E.R., E.R., M.J.H., S.L., P.S.; analysis: E.R., E.R., M.J.H., M.B., O.J., S.K.H., L.N.; writing manuscript: E.R., E.R., M.B., M.J.H., D.M., S.L., T.S., P.N. P.N. had full access to the data and is the guarantor of this work. All authors have read and agreed to the final version of the manuscript. E.R. and E.R. contributed equally.
Funding
The study was conducted within the Center of Excellence in Cardiovascular and Metabolic Diseases, supported by the Academy of Finland, the University of Turku, Turku University Hospital, Åbo Akademi University, Finnish Diabetes Foundation, Sigrid Jusélius Foundation. ER reports funding from the Emil Aaltonen Foundation, the Finnish Cultural Foundation, the Paulo Foundation, the Maud Kuistilan Muistosäätiö, the Paavo Nurmi Foundation, the Finnish Diabetes Research Foundation, from the Finnish Medical Foundation and from the European Foundation for the Study of Diabetes (EFSD).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Eleni Rebelos and Eero Rissanen.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-11557-2.
References
- 1.Ho AJ, et al. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol. Aging. 2010;31:1326–1339. doi: 10.1016/j.neurobiolaging.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rebelos E, Rinne JO, Nuutila P, Ekblad LL. Brain glucose metabolism in health, obesity, and cognitive decline-does insulin have anything to do with it? A narrative review. J. Clin. Med. 2021;10:1532. doi: 10.3390/jcm10071532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afshin A, et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raji CA, et al. Brain structure and obesity. Hum. Brain Mapp. 2010;31:353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum. Brain Mapp. 2010;31:1052–1064. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan A, et al. Neurofilaments form a highly stable stationary cytoskeleton after reaching a critical level in axons. J. Neurosci. 2009;29:11316–11329. doi: 10.1523/JNEUROSCI.1942-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalil M, et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018;14:577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 9.Lewczuk P, et al. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimers. Res. Ther. 2018;10:71. doi: 10.1186/s13195-018-0404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhle J, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92:e1007–e1015. doi: 10.1212/WNL.0000000000007032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lafrenaye AD, et al. Circulating GFAP and Iba-1 levels are associated with pathophysiological sequelae in the thalamus in a pig model of mild TBI. Sci. Rep. 2020;10:13369. doi: 10.1038/s41598-020-70266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oeckl P, et al. Glial fibrillary acidic protein in serum is increased in Alzheimer’s disease and correlates with cognitive impairment. J. Alzheimers. Dis. 2019;67:481–488. doi: 10.3233/JAD-180325. [DOI] [PubMed] [Google Scholar]
- 13.Högel H, et al. Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity. Mult. Scler. 2020;26:210–219. doi: 10.1177/1352458518819380. [DOI] [PubMed] [Google Scholar]
- 14.Tuulari JJ, et al. Bariatric surgery induces white and grey matter density recovery in the morbidly obese: A voxel-based morphometric study. Hum. Brain Mapp. 2016;37:3745–3756. doi: 10.1002/hbm.23272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kokubo Y, et al. Herniated and spondylotic intervertebral discs of the human cervical spine: histological and immunohistological findings in 500 en bloc surgical samples. Laboratory investigation. J. Neurosurg. Spine. 2008;9:285–295. doi: 10.3171/SPI/2008/9/9/285. [DOI] [PubMed] [Google Scholar]
- 16.Mäkinen J, et al. Obesity-associated intestinal insulin resistance is ameliorated after bariatric surgery. Diabetologia. 2015;58:1055–1062. doi: 10.1007/s00125-015-3501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buse JB, et al. How do we define cure of diabetes? Diabetes Care. 2009;32:2133–2135. doi: 10.2337/dc09-9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224–232. [PubMed] [Google Scholar]
- 20.Lemmens HJM, Bernstein DP, Brodsky JB. Estimating blood volume in obese and morbidly obese patients. Obes. Surg. 2006;16:773–776. doi: 10.1381/096089206777346673. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5:303. [PubMed] [Google Scholar]
- 23.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol. Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Middeldorp J, Hol EM. GFAP in health and disease. Prog. Neurobiol. 2011;93:421–443. doi: 10.1016/j.pneurobio.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Thaler JP, et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rebelos E, et al. Brain free fatty acid uptake is elevated in morbid obesity, and is irreversible 6 months after bariatric surgery: A positron emission tomography study. Diabetes. Obes. Metab. 2020;22:1074–1082. doi: 10.1111/dom.13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebelos E, et al. Brain glucose uptake is associated with endogenous glucose production in obese patients before and after bariatric surgery and predicts metabolic outcome at follow-up. Diabetes. Obes. Metab. 2019;21:218–226. doi: 10.1111/dom.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rebelos E, et al. Insulin resistance is associated with enhanced brain glucose uptake during euglycemic hyperinsulinemia: A large-scale PET cohort. Diabetes Care. 2021;44:1–7. doi: 10.2337/dc20-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manouchehrinia A, et al. Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann. Clin. Transl. Neurol. 2020;7:139–143. doi: 10.1002/acn3.50972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebelos E, et al. Renal hemodynamics and fatty acid uptake: Effects of obesity and weight loss. Am. J. Physiol. Endocrinol. Metab. 2019;317:E871–E878. doi: 10.1152/ajpendo.00135.2019. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int. 2008;73:19–33. doi: 10.1038/sj.ki.5002586. [DOI] [PubMed] [Google Scholar]
- 32.Akamine S, et al. Renal function is associated with blood neurofilament light chain level in older adults. Sci. Rep. 2020;10:20350. doi: 10.1038/s41598-020-76990-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: An emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 34.Tojo A, Kinugasa S. Mechanisms of glomerular albumin filtration and tubular reabsorption. Int. J. Nephrol. 2012;2012:481520. doi: 10.1155/2012/481520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adiutori R, et al. The proteome of neurofilament-containing protein aggregates in blood. Biochem. Biophys. Rep. 2018;14:168–177. doi: 10.1016/j.bbrep.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MK, et al. Glial fibrillary acidic protein is expressed in the aged rat olfactory epithelium. Acta Otolaryngol. 2005;125:883–887. doi: 10.1080/00016480510031542. [DOI] [PubMed] [Google Scholar]
- 37.Khalil M, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat. Commun. 2020;11:812. doi: 10.1038/s41467-020-14612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdelhak A, Huss A, Kassubek J, Tumani H, Otto M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci. Rep. 2018;8:14798. doi: 10.1038/s41598-018-33158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vågberg M, et al. Levels and age dependency of neurofilament light and glial fibrillary acidic protein in healthy individuals and their relation to the brain parenchymal fraction. PLoS One. 2015;10:e0135886. doi: 10.1371/journal.pone.0135886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heller C, et al. Plasma glial fibrillary acidic protein is raised in progranulin-associated frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry. 2020;91:263–270. doi: 10.1136/jnnp-2019-321954. [DOI] [PubMed] [Google Scholar]
- 41.Liddelow SA, Barres BA. Reactive astrocytes: Production, function, and therapeutic potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Allen NJ, Barres BA. Neuroscience: Glia—more than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- 43.González-García I, García-Cáceres C. Hypothalamic astrocytes as a specialized and responsive cell population in obesity. Int. J. Mol. Sci. 2021;22:6176. doi: 10.3390/ijms22126176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.