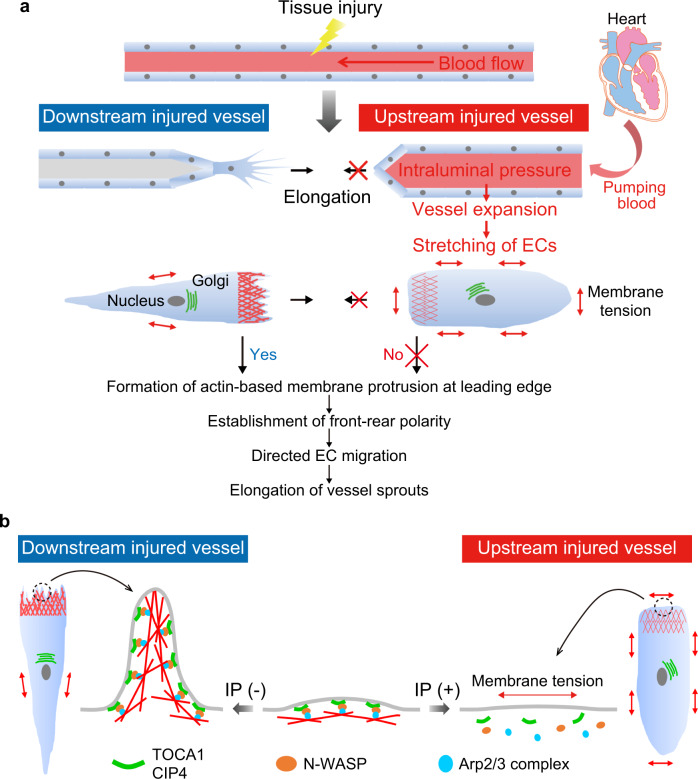
Fig. 10. Schematic representation of proposed model accounting for how blood flow-driven intraluminal pressure regulates angiogenesis during wound healing.
a When blood vessels are damaged by tissue injury, elongation of severed blood vessels is actively induced at sites downstream from blood flow, whereas vessels located upstream do not elongate efficiently. ECs in downstream injured vessels extend leading-edge protrusions and establish front–rear polarity to directionally migrate and elongate vessel sprouts. In clear contrast, in upstream injured vessels, IP load induces vessel expansion and EC stretching, inhibiting actin-based protrusion and front–rear polarization, thereby impairing vessel elongation. b In downstream injured vessels, TOCA1 and CIP4 bind to plasma membranes at the leading edge of ECs and recruit N-WASP and Arp2/3 complexes to induce actin-based membrane protrusion, promoting directional EC migration and vessel elongation. However, in upstream injured vessels, IP load-induced EC stretching prevents leading edge localization of TOCA1 and CIP4, thereby inhibiting actin-based protrusion and front–rear polarization, thus impairing vessel elongation.