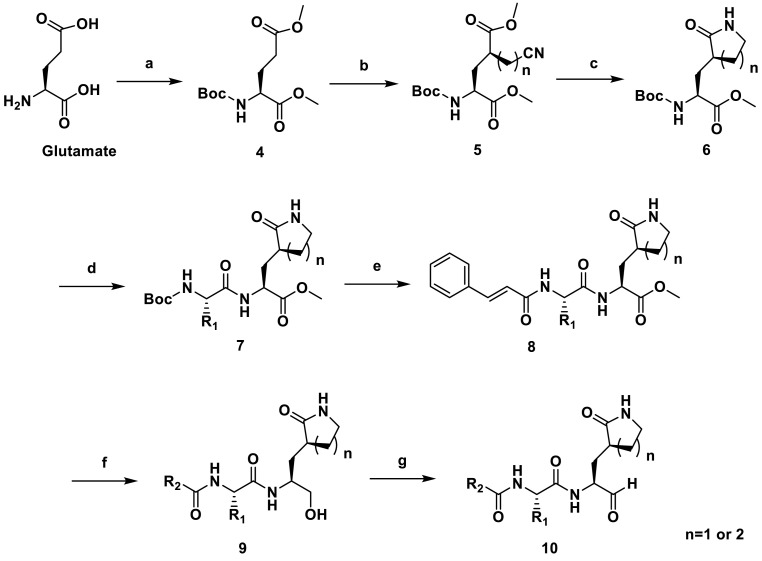
Scheme 1.
Synthetic Scheme of 10a-10l. Reagents and conditions: (a) (1) SOCl2, MeOH, reflux, 3 h, (2) Di-tert-butyl pyrocarbonate, TEA, THF, 25 °C, 12 h; yield: 98.4% for two steps; (b) (1) LiHMDS, anhydrous THF, −78 °C, 3 h, argon atmosphere; (2) 2-Bromoacetonitrile or 3-Bromopropionitrile (dissolved in anhydrous THF), −78 °C, 1.5 h; yield: 55.4%–59% for two steps; (c) CoCl2. 6H2O, NaBH4, MeOH, 0 °C, 48 h, yield: 46.3%; (d) (1) TFA, anhydrous DCM, 25 °C, 3 h, add TEA to adjust pH to 7.0; (2) various Boc-protected amino acid, EDCI, HOBt and TEA, anhydrous DCM, 25 °C, 12 h; 47%–65% for two steps; (e) (1) TFA, anhydrous DCM, RT, 3 h, add TEA to adjust pH to 7.0; (2) Cinnamic acid, EDCI, HOBt and TEA, anhydrous DCM, 25 °C, 12 h, 32–44%. (f) NaBH4, MeOH, 0 °C–25 °C, 3 h, 56–77%; (g) Dess-Martin Reagent, DCM, 25 °C, 2 h, 71–91%.