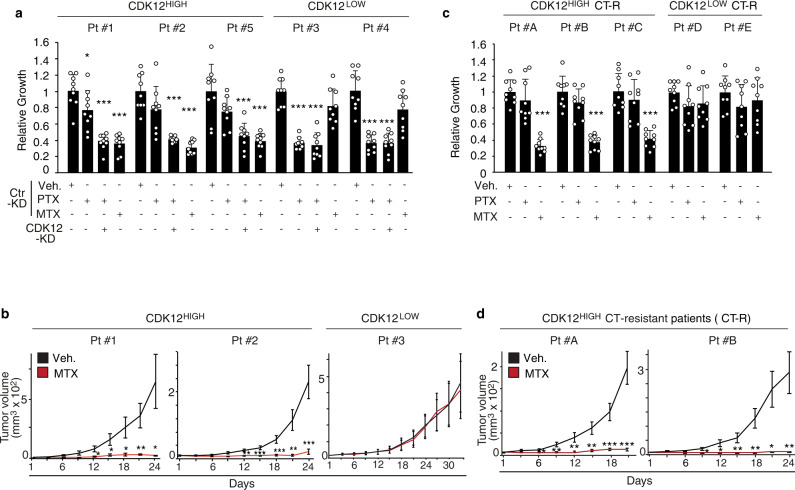
Fig. 10. Preclinical validation of the therapeutic actionability of SGOC metabolism alterations in CDK12-overexpressing human breast cancers.
a Three-days in vitro growth of CDK12HIGH (Patient #1, #2 and #5) vs. CDK12LOW (Patient #3 and #4) PDX cells control silenced (Ctr-KD) or silenced for CDK12 (CDK12-KD), in response to treatment with paclitaxel (PTX, 20 nM), methotrexate (MTX, 1 μM), PTX plus CDK12-KD, and vehicle (Veh), as a control. Data are expressed as relative to day 3 in each condition and are the mean ± SD (n = 3). *P = 0.035, ***P < 0.001, relative to vehicle in each condition, two-sided unpaired t-test. b In vivo growth kinetics of CDK12HIGH (Pt#1 and #2) and CDK12LOW (Pt#3) PDXs transplanted in the mammary fat pads of NSG mice administered with vehicle control (Veh.) or methotrexate (MTX, 10 mg/kg) (n ≥ 4 tumors per experimental group). Data are the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001, relative to vehicle-treated mice, two-sided unpaired t-test. c Three-days in vitro growth of cells derived from taxanes/anthracycline chemotherapy-resistant (CT-R) CDK12HIGH (Patients #A, #B and #C) and CDK12LOW (Patients #D and #E) PDXs, in response to treatment with paclitaxel (PTX, 20 nM), methotrexate (MTX, 1 μM) or vehicle control (Veh.) for 72 h. Data are expressed as relative to day 3 in each condition and are the mean ± SD (n = 3). ***P < 0.001 relative to vehicle in each condition, two-sided unpaired t-test. d In vivo growth kinetics of two tumor xenografts derived from taxane/antracycline-resistant CDK12HIGH PDX cells (CT-R) (Patient #A and #B) transplanted in NSG mice administered with vehicle control (Veh) or methotrexate (MTX, 10 mg/kg) (n ≥ 8 tumors/per experimental group). Data are the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001, relative to matching controls, two-sided unpaired t-test. Source data are provided as Source Data file.