Figure 4. Deconvolved zero-charge mass spectra after quadrupole isolation and HCD dissociation of a 2(POP5·RPP30) charge state.
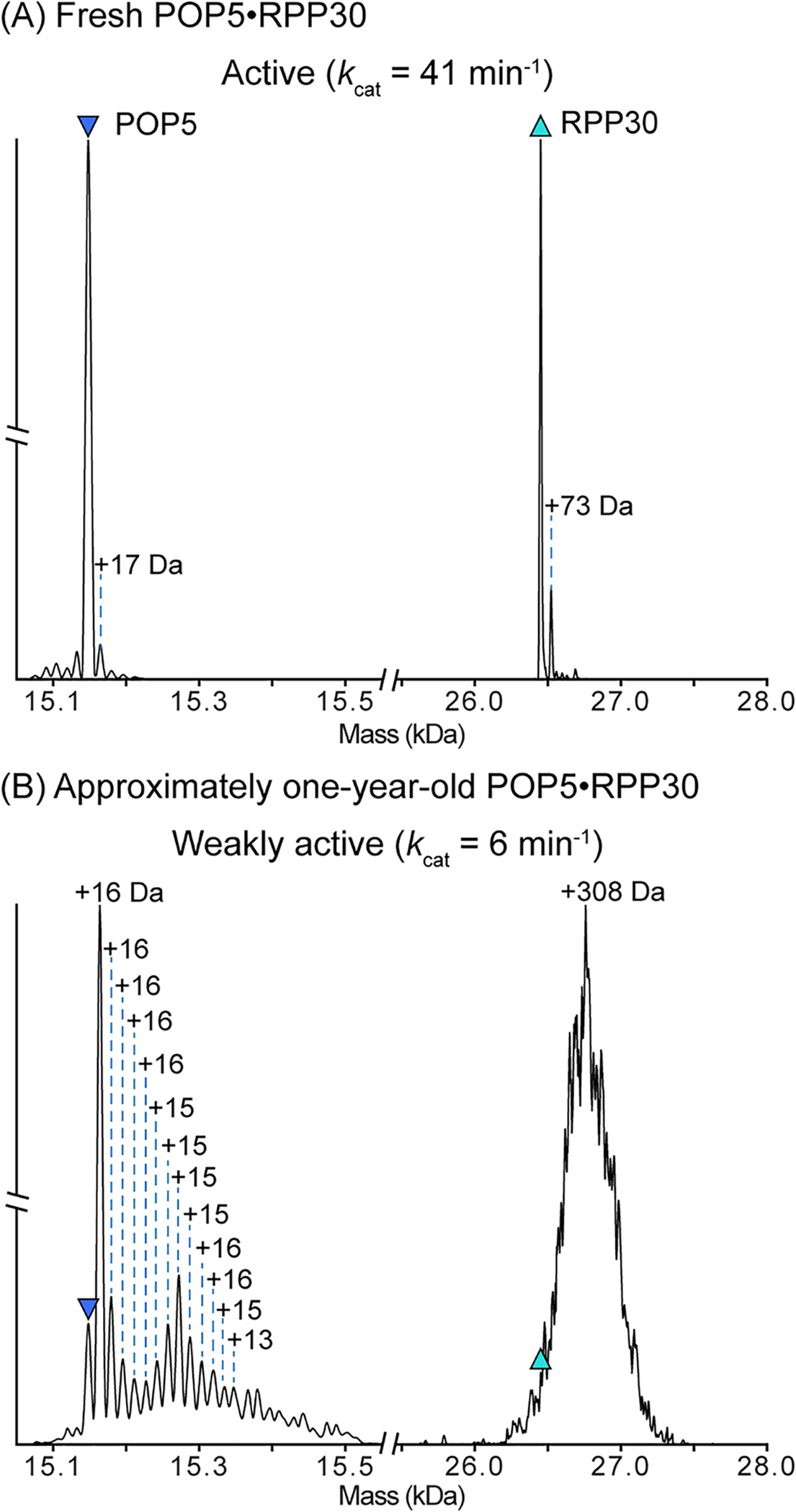
Dissociation of the same preparation of POP5·RPP30 within (A) one month and (B) approximately one year after overexpression and purification. Purified POP5·RPP30 is stored at −20°C as described in 3.3.1. Prior to analysis, POP5·RPP30 was dialyzed into 500 mM ammonium acetate. After one month (A), nearly all of the POP5 and RPP30 have the expected mass, indicating the majority of the protein in solution is unmodified. However, after one year (B), both proteins in the complex are heavily modified. The major POP5 peak now shows a single oxidation (+16 Da), and the presence of multiple additional masses correspond to further oxidations. Observed peak overlap and the peak distribution suggest that POP5 likely has other adducts and/or modifications that cannot be assigned. Similarly, the molecular weight of RPP30 has shifted by an average of +308 Da. The unresolved peaks suggest heterogeneous mass additions, which may include modifications, such as oxidation, and other tightly-bound adducts. M. maripaludis POP5 contains five Cys, Met, and Trp residues, which are all readily oxidized (Raftery, 2014). RPP30 has eight such residues. Thus, oxidation is a likely route for modification of these two protein subunits (RPP21 and RPP29 are similarly modified; data not shown). As indicated by the kcat values above each spectrum, the activity of M. maripaludis significantly decreases over time, perhaps due to modification of the protein subunits during storage.
