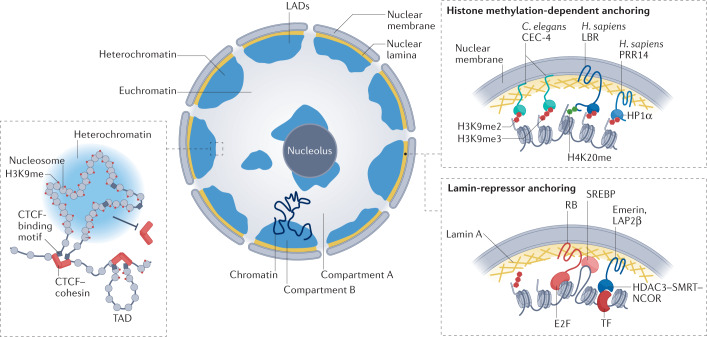
Fig. 2. Roles of H3K9 methylation in long-range chromatin interactions and nuclear organization.
An interphase nucleus in a differentiated vertebrate tissue is shown with heterochromatin (blue) sequestered against the nucleolus and against the nuclear lamina.The separation of active and inactive chromatin domains can manifest itself as lamin-associated domains (LADs)207 or as A and B compartments208 (Box 2). B compartments correspond to gene-inactive chromatin (heterochromatin) and largely include LADs. A compartments correspond to gene-active chromatin, which is further organized into topologically associating domains (TADs; left), which enable enhancer–promoter interaction that stimulates gene expression (not shown). In differentiated tissues, methylated histone H3 Lys9 (H3K9me) blocks TAD formation by preventing the binding of CCCTC-binding factor (CTCF) and cohesin complexes, which delineate TAD borders. On the right are shown several mechanisms for heterochromatin anchorage at the nuclear periphery. In Caenorhabditis elegans, the nuclear envelope-associated protein CEC-4 recognizes all forms of H3K9me (monomethylated H3K9 (H3K9me1), dimethylated H3K9 (H3K9me2) and trimethylated H3K9 (H3K9me3)) through its chromodomain, with affinity roughly equal to that of heterochromatin protein 1 (HP1)209. CEC-4 contributes directly to heterochromatin anchoring in worm embryos209–211, but upon tissue differentiation, as in mammals, additional anchoring pathways are induced, which in worms are independent of H3K9me212. Anchoring mechanisms present in differentiated mammalian cells include a histone methylation-dependent pathway with at least two potential anchors: proline-rich protein 14 (PRR14; potentially a functional homologue of CEC-4) is a non-transmembrane protein that anchors heterochromatin through H3K9me and HP1, and lamin B receptor (LBR), which is a transmembrane factor that binds both HP1α and dimethylated histone H4 Lys20 (H4K20me2) through its Tudor domain213–216. Another mechanism is lamin A dependent214 and likely involves its interaction with RB and/or with transcription factors (TFs), such as SREBP. Emerin and lamina-associated polypeptide 2, isoform-β (LAP2β) are additional lamin-associated factors that bind chromatin through the histone deacetylase 3 (HDAC3)–nuclear receptor co-repressor 1 (NCOR)–silencing mediator of retinoic acid and thyroid hormone receptor (SMRT; also known as NCOR2) complex and potentially through tissue-specific transcription factors215,216 (Box 2). H. sapiens, Homo sapiens.