Abstract
Cymbopogon winterianus, known as “citronella grass”, is an important aromatic and medicinal tropical herbaceous plant. The essential oil of C. winterianus (EOCw) is popularly used to play an important role in improving human health due to its potential as a bioactive component. The present study aimed to identify the components of the essential oil of C. winterianus and verify its leishmanicidal and trypanocidal potential, as well as the cytotoxicity in mammalian cells, in vitro. The EOCw had geraniol (42.13%), citronellal (17.31%), and citronellol (16.91%) as major constituents. The essential oil only exhibited significant cytotoxicity in mammalian fibroblasts at concentrations greater than 250 μg/mL, while regarding antipromastigote and antiepimastigote activities, they presented values considered clinically relevant, since both had LC50 < 62.5 μg/mL. It can be concluded that this is a pioneer study on the potential of the essential oil of C. winterianus and its use against the parasites T. cruzi and L. brasiliensis, and its importance is also based on this fact. Additionally, according to the results, C. winterianus was effective in presenting values of clinical relevance and low toxicity and, therefore, an indicator of popular use.
Keywords: C. winterianus, geraniol, cytotoxicity, leishmanicidal, trypanocidal
1. Introduction
Neglected tropical diseases (NTDs) are a diverse group of tropical infections common in low-income populations in developing regions of Africa, Asia, and the Americas. Two of the main pathologies that fall into this category are Mucocutaneous leishmaniasis and Trypanosomiasis, caused by the protozoa Leishmania braziliensis and Trypanosoma cruzi, respectively, both belonging to the Trypanosomatidae family, class Kinetoplastea. Treatments for such infections are expensive, so it has been estimated that controlling NTDs would require between USD 2 billion and USD 3 billion in funding over the next five-to-seven years [1,2].
Mucocutaneous leishmaniasis, caused by the parasite L. braziliensis, leads to the partial or total destruction of the mucous membranes of the nose, mouth, and throat. More than 90% of cases of Mucocutaneous leishmaniasis occur in Bolivia, Brazil, Ethiopia, and Peru [3]. The use of chemotherapeutics, such as miltelfosine, and pentavalent antimonials, such as N-methyl-glucamine antimoniate, is often the first step in the treatment of Leishmania infections [4]. Recent evaluations have shown increased resistance to antimonials in some endemic areas, limiting their effectiveness and demanding a more species-specific solution when possible [5]. Two pentavalent antimony products available in the US are sodium stibogluconate and meglumine antimoniate. The recommended dosage for both is 20 mg/kg/day for 28 to 30 days in mucocutaneous and visceral leishmaniasis [6].
Chagas disease is caused by the protozoan T. cruzi transmitted by the feces of triatomines, which are contaminated with metacyclic trypomastigotes and ejected by the insect after feeding [7]. T. cruzi invades and multiplies as amastigotes within many different types of host cells, including muscle cells, macrophages, and fibroblasts [8]. Chagas disease is common in parts of Mexico, Central America, and South America, where an estimated 8 million people are infected [9]. Current options for the treatment of Chagas disease are restricted to benznidazole and nifurtimox, which are not well tolerated and imply frequent discontinuation of treatment [10].
In this scenario, there are essential oils (EOs) from several plant species with antiprotozoal properties that have become potential chemotherapeutic agents [11,12]. EOs are insoluble in inorganic solvents (water) but soluble in organic solvents (ether, alcohol, fixed oils). They are volatile liquids, with a characteristic odor, and are widely used in the perfumery, aromatherapy, and cosmetics industry; they are also increasingly important in the pharmaceutical scene due to their wide range of applications and biological activities [13].
Java citronella (Cymbopogon winterianus Jowitt ex Bor.) is an aromatic grass belonging to the Poaceae family that provides essential oils by stem distillation. It is widely used as a source in perfumery, soap, cosmetics, and flavoring industries. Leaf-blades are linear, tapering gradually to a long, membranous, acuminate shape, and up to 1 m long and 1.5 cm wide. C. winterianus is an industrially important perennial multi-crop cultivated in parts of tropical and subtropical areas of Asia, Africa, and America [14,15].
The study of C. winterianus in this research is due to its varied popular use and its diverse biological activities. Therefore, the present study aimed to identify the components and verify the leishmanicidal and trypanocidal power, as well as the in vitro cytotoxicity of the essential oil C. winterianus (EOCw).
2. Results
The chromatographic profile (chromatogram) of EOCw (Figure 1) revealed by gas chromatography with mass spectrometry (GC/MS), indicates a single substance with retention time = 31.299 min and maximum detection intensity (most concentrated). As can be seen in Table 1, the chemical composition of the EOCw presented as major constituents in ascending order of concentration: citronellol (16.91%), citronellal (17.31%), and geraniol (42.13%), followed by other compounds in lower concentrations.
Figure 1.
GC/MS chromatogram of the essential oil of C. winterianus with total ion current (TIC) peak reports. * Total Íon Current.
Table 1.
Chemical composition (%) of the essential oil of C. winterianus.
| Components | RT (min) a | (%) |
|---|---|---|
| Limonene | 17.88 | 4.24 |
| Citronellal | 25.27 | 17.31 |
| Citronellol | 29.70 | 16.91 |
| Geraniol | 31.29 | 42.13 |
| β-elemene | 39.37 | 2.69 |
| δ-Cadinene | 46.65 | 1.05 |
| Elemol | 47.24 | 6.71 |
| Germacrene | 47.80 | 4.44 |
| Guaiol | 48.87 | 1.14 |
| Nerolidol | 52.70 | 3.38 |
| Total | 100.00 |
a Retention time.
In Figure 2, it is graphically observed that the cytotoxic activity of the EOCw had a relatively high LC50 of 96.56 μg/mL, which is desired for products intended for human consumption.
Figure 2.
Cytotoxicity of the essential oil of C. winterianus, with the confidence interval for oil at 95% (87.07–107.10). LC50 was obtained through non-linear regression of means.
Table 2 shows, from a different perspective, the same promising result observed in Figure 2, which would be the low cytotoxicity exhibited by EOCw against mammalian cells that would normally be infected by the parasites; cell damage only showed to be significant from the concentration of 125 µg/mL.
Table 2.
Survival of fibroblasts exposed to the essential oil of C. winterianus.
| Natural Product | Conc. µg/mL | %C | ±%DS |
|---|---|---|---|
| C. winterianus | 1000 | 0 | – |
| 500 | 0 | – | |
| 250 | 2.15 | 0.49 | |
| 125 | 35.79 | 0.80 | |
| 62.5 | 89.96 | 0.70 | |
| 31.5 | 90.96 | 0.77 |
According to Figure 3 and Figure 4, the EOCw presented inhibition values considered very promising for the promastigote and epimastigote forms of L. brasiliense and T. cruzi, with LC50 values of 44.98 μg/mL and 72.60 μg/mL, for Leishmania and Trypanosoma, respectively, with performance equivalent to that of Pentamidine, at a concentration of 125 μg/mL.
Figure 3.
Cytotoxicity of the promastigote L. brasiliensis treated with essential oil of C. winterianus. LC50, with the confidence interval for oil at 95% (35.55–56.92). LC50 was obtained through non-linear regression of means.
Figure 4.
Cytotoxicity of the epimastigote T. cruzi treated with essential oil of C. winterianus. LC50 confidence interval for oil is 95% (50.63–104.10). LC50 was obtained through non-linear regression of means.
Additionally, observing Table 3 and Table 4, it can be inferred that the EOCw showed survival percentage values considered very promising for the promastigote and epimastigote forms of L. brasiliense and T. cruzi, respectively.
Table 3.
Survival of the promastigote L. brasiliensis treated with the essential oil of C. winterianus.
| Natural Product | Conc. µg/mL C. winterianus |
%S | ±%DS | Conc. µg/mL Pentamidine |
%S | ±%DS |
|---|---|---|---|---|---|---|
| C. winterianus | 1000 | 0 | - | |||
| 500 | 0 | - | ||||
| 250 | 0 | - | ||||
| 125 | 0 | - | ||||
| 100 | 5.7 | 0.2 | ||||
| 62.5 | 39.13 | 2.11 | ||||
| 31.5 | 65.56 | 1.02 | ||||
| 25 | 10.7 | 0.4 | ||||
| 6.2 | 40.5 | 0.2 | ||||
| 3.2 | 83.6 | 0.9 |
Table 4.
Survival of the epimastigote T. cruzi treated with the essential oil of C. winterianus.
| Natural Product | Conc. µg/mL C. winterianus |
%S | ± %DS | Conc. g/mL Pentamidine |
%S | ±%DS |
|---|---|---|---|---|---|---|
| C. winterianus | 1000 | 0 | – | |||
| 500 | 0 | – | ||||
| 250 | 0 | – | ||||
| 125 | 0 | – | ||||
| 100 | 0 | 0.7 | ||||
| 62.5 | 74.89 | 1.70 | ||||
| 50 | 6.6 | 0.5 | ||||
| 31.5 | 83.88 | 2.52 | ||||
| 10 | 15.4 | 0.6 | ||||
| 1.0 | 56.3 | 0.5 | ||||
| 0.5 | 85.4 | 0.6 | ||||
| 0.1 | 99.6 | 0.3 |
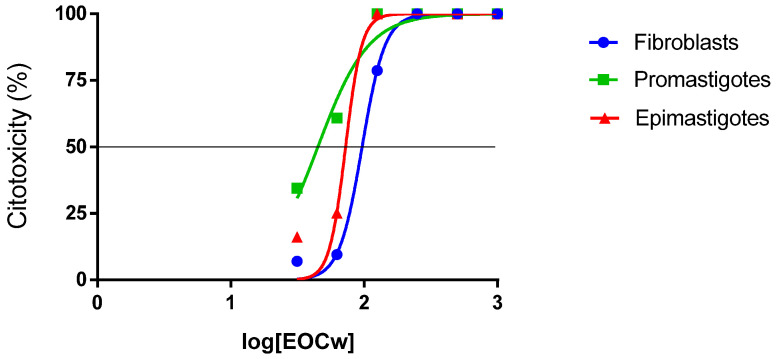
Figure 5 shows the log-dose vs. cytotoxicity curves of EOCw for fibroblasts, promastigotes, and epimastigotes estimated through non-linear regression of means, demonstrating that the anti-kinetoplastid effect was more intense than the cytotoxic effect in fibroblasts.
Figure 5.
Log-dose vs. cytotoxicity curves for NCTC 929 fibroblasts, L. braziliensis promastigotes, and T. cruzi epimastigotes were estimated by non-linear regression of means.
3. Discussion
More than 200 different components present in pure essential oils have been reported. These mixtures typically contain phenylpropanoic derivatives or terpenes [16]. In general, gas chromatography (GC) is used for the analysis of volatile constituents present in essential oils, and liquid chromatography (LC) is used for the analysis of non-volatile constituents [17]. Usually, most essential oils are composed of volatile fractions, which contain monoterpenes, sesquiterpenes, and their oxygenated derivatives, where aliphatic alcohols, esters, and aldehydes may also be present [18,19].
The main compound found in the essential oil of C. winterianus using the GC technique with mass spectrometry was geraniol (3,7-dimethyl-2,6-octadien-8-ol) (42.13%), an acyclic monoterpenic alcohol in which biosynthesis occurs mainly in the cytosol of glandular trichomes via geranyl monophosphate (GP) through the action of a Nudix hydrolase [20,21]. Secondarily, citronellal (17.31%) and citronellol (16.91%) were detected in median concentrations, in which biosynthesis is related to the expression of heterodimeric geranyl diphosphate synthases (GPPS-SSU) and plastid geranylgeranyl diphosphate (GGPP) [22].
Essential oils of Cymbopogon spp. are diverse in chemical composition and have many bioactivities and potentials of great pharmaceutical and medicinal significance [23]. In this genus, it is possible to observe a remarkable variation in the composition and yield of the essential oil, which varies from 0.3% in C. travancorensis to 1.2% in C. martinii. The main components of citral essential oil “a” and “b” were detected in Cymbopogon pendulus, C. flexuosus, and C. citratus with the largest in C. citratus. Cymbopogon confertiflorus and C. nardus var. confertiflorus present an essential oil with a high content of geraniol (67.7% and 46.0%, respectively), and another group including C. nardus var. nardus, C. nardus var. Java II, and C. winterianus have less geraniol in their essential oil (ranging from 20% to 25%) [24,25].
Regarding the composition of the EOCw, the results obtained in the present study differ slightly from those already observed in the literature, according to which the proportion of citronellal (up to 40.23%) was higher than that of geraniol (up to 22.78%), with citronellol always having the third-highest proportion [26,27]. In the study by Kakaraparthi et al. [28], it is possible to observe that the proportion of geraniol has a significant positive correlation (0.60) with the maximum temperature to which the plant is exposed.
Studies that evaluated the cytotoxic effect of essential oils of species of the Cymbopogon genus on mammalian cells showed that these are usually similar to that observed in the present study for C. winterianus (LC50 = 96.56 µL/mL) (Figure 2 and Figure 5, Table 2) so that the LC50 varies in the range of 50–300 µL/mL, where an LC50 > 50 µL/mL is indicated as non-cytotoxic [29,30]. In fact, in Cymbopogon spp. with a similar proportion of constituents to that observed here in EOCW, a cytoprotective effect is observed [31]. In the literature, a great variation in the antioxidant capacity of EOCw can be observed (IC50 = 12. 66 ± 0.56/743 ± 18 μg/mL), especially with regard to its capacity to reduce the 2,2-diphenyl radical -1-picrylhydrazyl (DPPH), which may be related to its great variability in its composition [32,33].
Essential oil of C. winterianus, as well as that of C. citratus, has already been shown to inhibit cytotoxicity in murine neutrophils through a mechanism not related to free radical scavenging [34]. The low cytotoxicity observed here (Figure 2 and Figure 5, Table 2) is probably due to the absence of compounds known to be toxic to mammalian cells in EOCw (Table 1), such as citral [35]. In the study by Sinha et al. [36], a weak cytotoxic effect of citronella essential oil on human lymphocytes was observed, although the use of geraniol had the opposite effect.
Many human cell lines did not show genotoxic or clastogenic/aneugenic effects when exposed to high concentrations (100 μg/mL) of geraniol [37]. It has been shown that geraniol, despite its mild cytotoxicity in human lymphocytes, is able to scavenge free radicals similarly to butylated hydroxytoluene (BHT), ascorbic acid, and α-tocopherol, which are potent antioxidants [38]. Regarding citronellal, the second most-abundant compound in EOCw (Table 1), it is known to be non-toxic to mammalian cells (LC50 > 50 μg/mL) [30].
Few studies reporting the leishmanicidal effects of essential oils from species of the Cymbopogon genus have been reported, the majority of which cite the oil of C. citratus as a potent leishmanicidal agent, with LC50 values = 25 μg/mL and 1.7 µg/mL for Leishmania infantum and L. amazonensis, respectively, where the latter was superior to the pentamidine drug used here [39,40]. The leishmanicidal effects of EOCw observed here (Figure 3 and Figure 5, Table 3) are almost certainly due to a large number of oxygenated terpenes present in its composition (Table 1), which, as in C. citratus, demonstrate a strong leishmanicidal effect, especially for L. braziliensis [41].
Geraniol, identified here in large quantities in the EOCw (Table 1), did not show antipromastigote activity against L. braziliensis at a concentration of 100 µg/mL in the study by Carneiro et al. [42]; however, in the same study, it was shown that citronellal has excellent antipromastigote activity. Other plant species whose essential oils contain major amounts of citronellal, such as Eucalyptus citriodora, also exhibit good leishmanicidal effects [43]. Different species of Leishmania seem to be significantly affected by geraniol, which, in these cases, has LC50 = 3.78 µg/mL and 5.57 µg/mL for L. infantum and L. major, respectively [44].
In vitro analyses using molecular docking demonstrated that geraniol is strongly anchored by the molecules of L. major uridine diphosphate–glucose pyrophosphorylase (LmajUGPase), L. major methionyl t-RNA synthetase (LmajMetRS), and L. infantum nicotinamidase (LinfPnC1) [45]. The same type of evaluation indicated the spermidine synthase (SpdS) enzyme as a possible anchoring site for geraniol in L. donovani; however, linalool, with similar potential, does not present a good leishmanicidal effect [46,47]. Citral, molecularly similar to geraniol, is capable of causing considerable ultrastructural changes in Leishmania spp., including mitochondrial and kinetoplast swelling, autophagosomal structures, nuclear membrane rupture, and nuclear chromatin condensation, which would justify the antipromastigote effect observed here (Figure 3 and Figure 5, Table 3) [39]. Exposure to high concentrations of eugenol, another oxygenated monoterpene, also caused extensive fatal cell damage in Leishmania spp. promastigotes, such as cells with two or more flagella, swollen mitochondria and altered inner mitochondrial membrane, with a significant increase in the number of cristae, indicating an associated mechanism of action. to mitochondrial damage [48].
The performance of EOCw in inhibiting the epimastigote form of Trypanosoma cruzi (Figure 4 and Figure 5, Table 4) was similar to that observed in studies of the same scope using species of the genus Cymbopogon [49,50]. Cymbopogon citratus has already demonstrated a potent trypanocidal effect (LC50 = 3.2 μg/mL) against Trypanosoma brucei, that is, almost as efficient as the standard drug pentamidine used in our study, where its major component, citral, was found to be responsible for this performance, presenting a similar effect (LC50 = 18.9 μg/mL) [51].
Different forms of T. cruzi have already been shown to have different sensitivities to oxygenated monoterpenes such as geraniol and citronellal; trypomastigote forms are much more susceptible to its cytotoxic effects than epimastigotes [52]. Citral, abundant in Cymbopogon citratus essential oil, exhibits an exceptional trypanocidal effect on epimastigotes, presenting an LC50 = 42 μg/mL for T. cruzi, which is not observed in different preparations such as methanol extracts (68.25 μg/mL), probably due to the low concentration of citral [53,54,55].
One study observed that the antiproliferative effect of C. citratus essential oil was derived from its main constituent (citral) in the three evolutionary forms of T. cruzi (LC50/24 h < 50 μg/mL for citral), which, based on the ultrastructural analysis, induce cytoplasmic and nuclear extraction, while the plasma membrane remained morphologically preserved in the parasites [56]. T. cruzi cells exposed to oxygenated monoterpenes such as geraniol and citral showed typical characteristics of apoptosis, such as cytoplasmic bubble, cell shrinkage, absence of flagellum, loss of mitochondrial membrane potential, nuclear chromatin condensation, and DNA fragmentation probably due to loss of mitochondrial function [57,58].
4. Materials and Methods
4.1. Plant Material, Selection, and Identification
Cymbopogon winterianus leaves were collected in the morning at the Medicinal Plants Garden of the Regional University of Cariri (URCA), Crato, Ceará, Brazil, and authenticated by Prof. Afranio Fernandes at the Department of Biology at the Federal University of Ceará. Specimens of the plant are deposited at the Herbarium Prisco Bezerra, Fortaleza, Ceará, Brazil, voucher n° 43.194.
4.2. Obtaining Essential Oil from C. winterianus
Fresh leaves were cut into pieces and then washed and macerated with 99.9% ethanol for 72 h at room temperature. The essential oil was obtained in a Clevenger apparatus by hydrodistillation. Fresh leaves of C. winterianus were placed in a 5 L flask, together with 3 L of distilled water, and heated for 2 h. Then, the obtained mixture was separated, and the essential oil of C. winterianus was treated with anhydrous sodium sulfate, filtered, and kept under refrigeration until the moment of analysis.
4.3. Essential Oil Chemical Identification
The mass detection method applied in this study (secondary electron multiplier with conversion dynode) has the highest sensitivity and is the most suitable technique for estimating concentrations [59]. Oil analysis was performed with a Shimadzu GC/MS apparatus—QP2010 series (GC/MS system): Rtx-5MS capillary column (30 m × 0.25 mm, film thickness of 0.25 μm); helium carrier gas at 1.5 mL/min; injector temperature 250 °C; detector temperature 290 °C; column temperature 60–180 °C to 5 °C/min, then 180–280 °C to 10 °C/min (10 min). The scan speed was 0.5 scan/sec from m/z 40 to 350, and the split ratio was 1:200. Injected volume was 1 µL of (25 µL (essential oil)/5 mL CHCl3) (1:200). The solvent cut-off time was 2.5 min. The mass spectrometer was operated using 70 eV of ionization energy. The identification of the individual components was based on their mass spectral fragmentation based on the NIST 08 mass spectral library, retention indices, and comparison with published data. The relative retention rates were disregarded, since the manufacturer of the chromatography equipment used (Shimadzu©) indicated that measurement errors would increase for target peaks located far from the reference peak, making it difficult to find a relationship with a chemical structure [60].
4.4. Antiparasitic Activity
4.4.1. Cell Lines Used
Strains of CL-B5 parasites (clone CL-B5) were used for in vitro evaluation of activity on T. cruzi [61]. Parasites transfected with the β-galactosidase gene from Escherichia coli (LacZ) were provided by Dr. F. Buckner through the Gorgas Memorial Institute (Panamá). The epimastigote forms were cultivated in tryptose liver infusion at 28 °C, supplemented with 10% fetal bovine serum (FBS), 10 U/mL of penicillin, and 10 μg/mL of streptomycin at pH 7.2 and incubated with different concentrations of essential oil (1000; 500; 250; 125; 62.5; 31.5 μg/mL) and collected during the exponential growth phase [62].
The in vitro antileishmanial activity was established using L. braziliensis promastigotes (MHOM/CW/88/UA301) at 26 °C, cultured in Schneider’s medium for insects, supplemented with 10% (v/v) of fetal serum heat-inactivated calf, 2% normal human urine (v/v) plus penicillin and streptomycin [62]. The forms were seeded and incubated with different concentrations of essential oil (1000; 500; 250; 125; 62.5; 31.5 μg/mL).
4.4.2. Reagents
Resazurin sodium substance was obtained from Sigma-Aldrich (St. Louis, MO, USA) and stored at 4 °C protected from light. Resazurin solution was prepared with 1% phosphate buffer, pH 7, and previously sterilized by filtration. Subsequently, Chlorophenol red-β-D-galactopyranoside (CPRG, Roche, Indianapolis, IN, USA) was dissolved in a 0.9% solution of Triton X-100 (pH 7.4). Penicillin G (Ern, SA, Barcelona, Spain), streptomycin (Reig Jofre SA, Barcelona, Spain), and dimethylsulfoxide (DMSO) were also used.
4.4.3. In Vitro Epimastigote Sensitivity Assay
Assays were performed as described by Vega et al. [63], with crops that did not reach the stationary phase. Epimastigote forms were seeded at 1 × 105 per mL in 200 μL, in 96-well microdilution plates, which were incubated at 28 °C for 72 h. Then, 50 µL of CPRG solution was added to give a final concentration of 200 µM. Plates were incubated at 37 °C for an additional 6 h. Absorbance reading was performed in a spectrophotometer at 595 nm. Concentrations were tested in triplicate. Each experiment was performed twice separately. The percentage of inhibition (% AE) was calculated as follows:
| % AE = [(AE_AEB)/(AC_ACB)] × 100 |
where AE = absorbance of the experimental group; AEB = compound blank; AC = absorbance control group; CBA = culture environment blank. The essential oil was previously dissolved in DMSO. The concentration of DMSO (dimethylsulfoxide) used to allow the solubility of the oil was not greater than 0.01%. The effectiveness of Pentamidine as a trypanocidal drug was also determined.
4.4.4. In Vitro Leishmanicidal Assay
The tests were performed according to Mikus and Steverding [64] with some modifications. Oil activity was evaluated in triplicate. Promastigote forms (2.5 × 105 parasites/well) were cultured in 96-well plastic plates. Samples were dissolved in dimethylsulfoxide (DMSO). Different dilutions of compounds up to 200 mL of the final volume were added. After 48 h at 26 °C, 20 µL of resazurin solution was added and the oxidation-reduction was measured at 570 to 595 nm. Percentages of antipromastigotes (AP%) were calculated. The effectiveness of the reference leishmanicidal drug pentamidine was also determined.
4.5. Fibroblasts Cytotoxic Assays
To measure cell viability in mammalian cells, a colorimetric assay with resazurin was used. NCTC 929 fibroblasts were seeded (5 × 104 cells/well) in 96-well flat-bottom microdilution plates with 100 µL of RPMI 1640 medium for 24 h at 37 °C and cultured in 5% CO2 for cells to adhere to the plates. The medium was replaced by different concentrations of essential oil (1000; 500; 250; 125; 62.5; 31.5 μg/mL) in 200 μL of medium and incubated for another 24 h. Growth controls were included. Then, a volume of 20 μL of 2 mM resazurin solution was added and the plates were placed in the incubator for another 3 h to assess cell viability. Resazurin reduction was determined by measuring the wavelength absorbance at 490 nm and 595 nm. Each concentration was tested three times. The cytotoxicity of each compound was estimated by calculating the percentage of cytotoxicity (%C).
4.6. Statistical Analysis
Results are expressed as mean ± standard error of the mean (SEM) of three independent experiments performed in triplicate. Concentrations capable of causing 50% lethality (LC50) were calculated by non-linear regression log-dose vs. mean ± standard error of the mean, using GraphPad Prism software version 6.0.
5. Conclusions
This study elucidated the composition and revealed, for the first time, the antiparasitic effect of the essential oil of C. winterianus, especially against L. braziliensis, but also against T. cruzi. Furthermore, we were able to determine a low cytotoxic effect of this natural product against mammalian cells, which enables possible applications in vivo. Therefore, future studies with the isolated constituents of the essential oil of C. winterianus need to be evaluated regarding the molecular mechanisms of interaction with kinetoplastid and human cells.
Acknowledgments
The authors would like to thank the Postgraduate Studies in Biological Sciences at the Federal University of Pernambuco, UFPE.
Author Contributions
Conceptualization, P.S.P. and T.G.S.; methodology, M.C.V.-G.; validation, M.R. and C.C.; formal analysis, M.C.V.-G., S.J.A.S. and A.J.M.; investigation, C.V.B.O.; writing—original draft preparation, A.E.D. and C.V.B.O.; writing—review and editing, P.S.P., T.G.S., A.S., R.N. and H.D.M.C.; visualization, T.G.S., P.W. and H.D.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compound are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hotez P.J. A Plan to Defeat Neglected Tropical Diseases. Sci. Am. 2010;302:90–96. doi: 10.1038/scientificamerican0110-90. [DOI] [PubMed] [Google Scholar]
- 2.Archibald J.M., Simpson A.G.B., Slamovits C.H., Margulis L., Melkonian M., Chapman D.J., Corliss J.O. In: Handbook of the Protists. Archibald J.M., Simpson A.G.B., Slamovits C.H., Margulis L., Melkonian M., Chapman D.J., Corliss J.O., editors. Springer International Publishing; Cham, Switzerland: 2017. [Google Scholar]
- 3.WHO Leishmaniasis. [(accessed on 3 August 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis.
- 4.Darcis G., Van der Auwera G., Giot J.-B., Hayette M.-P., Tassin F., Arrese Estrada J., Cnops L., Moutschen M., de Leval L., Leonard P. Recurrence of visceral and muco-cutaneous leishmaniasis in a patient under immunosuppressive therapy. BMC Infect. Dis. 2017;17:478. doi: 10.1186/s12879-017-2571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kedzierski L. Leishmaniasis. Hum. Vaccin. 2011;7:1204–1214. doi: 10.4161/hv.7.11.17752. [DOI] [PubMed] [Google Scholar]
- 6.Sundar S., Chakravarty J. Leishmaniasis: An update of current pharmacotherapy. Expert Opin. Pharmacother. 2013;14:53–63. doi: 10.1517/14656566.2013.755515. [DOI] [PubMed] [Google Scholar]
- 7.Gibson W. Handbook of the Protists. Springer International Publishing; Cham, Switzerland: 2016. Kinetoplastea; pp. 1–50. [Google Scholar]
- 8.Andrade L.O., Andrews N.W. The Trypanosoma cruzi–host-cell interplay: Location, invasion, retention. Nat. Rev. Microbiol. 2005;3:819–823. doi: 10.1038/nrmicro1249. [DOI] [PubMed] [Google Scholar]
- 9.CDC Chagas Disease: Epidemiology and Risk Factors. [(accessed on 3 August 2021)]; Available online: https://www.cdc.gov/parasites/chagas/epi.html.
- 10.Altcheh J., Castro L., Dib J.C., Grossmann U., Huang E., Moscatelli G., Pinto Rocha J.J., Ramírez T.E. Prospective, historically controlled study to evaluate the efficacy and safety of a new paediatric formulation of nifurtimox in children aged 0 to 17 years with Chagas disease one year after treatment (CHICO) PLoS Negl. Trop. Dis. 2021;15:e0008912. doi: 10.1371/journal.pntd.0008912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monzote L., Scherbakov A.M., Scull R., Satyal P., Cos P., Shchekotikhin A.E., Gille L., Setzer W.N. Essential Oil from Melaleuca leucadendra: Antimicrobial, Antikinetoplastid, Antiproliferative and Cytotoxic Assessment. Molecules. 2020;25:5514. doi: 10.3390/molecules25235514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rottini M.M., Amaral A.C.F., Ferreira J.L.P., Oliveira E.S.C., Silva J.R.d., Taniwaki N.N., Santos A.R.d., Almeida-Souza F., de Souza C.d.F., Calabrese K.D. Endlicheria bracteolata (Meisn.) Essential Oil as a Weapon Against Leishmania amazonensis: In Vitro Assay. Molecules. 2019;24:2525. doi: 10.3390/molecules24142525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch M.C., Cortelli S.C., McGuire J.A., Zhang J., Ricci-Nittel D., Mordas C.J., Aquino D.R., Cortelli J.R. The effects of essential oil mouthrinses with or without alcohol on plaque and gingivitis: A randomized controlled clinical study. BMC Oral Health. 2018;18:6. doi: 10.1186/s12903-017-0454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shasany A.K., Lal R.K., Patra N.K., Darokar M.P., Garg A., Kumar S., Khanuja S.P.S. Phenotypic and RAPD diversity among Cymbopogon winterianus Jowitt accessions in relation to Cymbopogon nardus Rendle. Genet. Resour. Crop Evol. 2000;47:553–559. doi: 10.1023/A:1008712604390. [DOI] [Google Scholar]
- 15.Katiyar R., Gupta S., Yadav K. Cymbopogon Winterianus: An Important Species for Essential Java Citronella Oil and Medicinal Value; Proceedings of the National Conference on Forest Biodiversity: Earth’s Living Treasure; 22 May 2011; pp. 115–118. [Google Scholar]
- 16.Rao V.P.S., Pandey D. Ph.D. Thesis. National Institute of Technology; Rourkela, India: 2006. Extraction of Essential Oil and Its Applications. [Google Scholar]
- 17.Zellner B.D.A., Dugo P., Dugo G., Mondello L. Handbook of Essential Oils. CRC Press; Boca Raton, FA, USA: 2015. Chapter 4: Natural Variability of Essential Oil Components; pp. 104–143. [Google Scholar]
- 18.Jurevičiūtė R., Ložienė K., Bruno M., Maggio A., Rosselli S. Composition of essential oil of lemon thyme (Thymus × citriodorus) at different hydrodistillation times. Nat. Prod. Res. 2019;33:80–88. doi: 10.1080/14786419.2018.1434642. [DOI] [PubMed] [Google Scholar]
- 19.Hanif M.A., Nisar S., Khan G.S., Mushtaq Z., Zubair M. Essential Oil Research. Springer International Publishing; Cham, Switzerland: 2019. Essential Oils; pp. 3–17. [Google Scholar]
- 20.Bergman M.E., Bhardwaj M., Phillips M.A. Cytosolic geraniol and citronellol biosynthesis require a Nudix hydrolase in rose-scented geranium (Pelargonium graveolens) Plant J. 2021;107:493–510. doi: 10.1111/tpj.15304. [DOI] [PubMed] [Google Scholar]
- 21.Christoforides E., Fourtaka K., Andreou A., Bethanis K. X-ray crystallography and molecular dynamics studies of the inclusion complexes of geraniol in β-cyclodextrin, heptakis (2,6-di-O-methyl)-β-cyclodextrin and heptakis (2,3,6-tri-O-methyl)-β-cyclodextrin. J. Mol. Struct. 2020;1202:127350. doi: 10.1016/j.molstruc.2019.127350. [DOI] [Google Scholar]
- 22.Gutensohn M., Orlova I., Nguyen T.T.H., Davidovich-Rikanati R., Ferruzzi M.G., Sitrit Y., Lewinsohn E., Pichersky E., Dudareva N. Cytosolic monoterpene biosynthesis is supported by plastid-generated geranyl diphosphate substrate in transgenic tomato fruits. Plant J. 2013;75:351–363. doi: 10.1111/tpj.12212. [DOI] [PubMed] [Google Scholar]
- 23.Ganjewala D. Cymbopogon essential oils: Chemical compositions and bioactivities. Int. J. Essent. Oil Ther. 2009;3:56–65. [Google Scholar]
- 24.Akhila A. Essential Oil-Bearing Grasses The genus Cymbopogon. CRC Press; London, UK: 2010. [Google Scholar]
- 25.Khanuja S.P.S., Shasany A.K., Pawar A., Lal R.K., Darokar M.P., Naqvi A.A., Rajkumar S., Sundaresan V., Lal N., Kumar S. Essential oil constituents and RAPD markers to establish species relationship in Cymbopogon Spreng. (Poaceae) Biochem. Syst. Ecol. 2005;33:171–186. doi: 10.1016/j.bse.2004.06.011. [DOI] [Google Scholar]
- 26.Beneti S.C., Rosset E., Corazza M.L., Frizzo C.D., Di Luccio M., Oliveira J.V. Fractionation of citronella (Cymbopogon winterianus) essential oil and concentrated orange oil phase by batch vacuum distillation. J. Food Eng. 2011;102:348–354. doi: 10.1016/j.jfoodeng.2010.09.011. [DOI] [Google Scholar]
- 27.Simic A., Rančic A., Sokovic M.D., Ristic M., Grujic-Jovanovic S., Vukojevic J., Marin P.D. Essential Oil Composition of Cymbopogon winterianus and Carum carvi. and Their Antimicrobial Activities. Pharm. Biol. 2008;46:437–441. doi: 10.1080/13880200802055917. [DOI] [Google Scholar]
- 28.Kakaraparthi P.S., Srinivas K.V.N.S., Kumar J.K., Kumar A.N., Rajput D.K., Sarma V.U.M. Variation in the essential oil content and composition of Citronella (Cymbopogon winterianus Jowitt.) in relation to time of harvest and weather conditions. Ind. Crops Prod. 2014;61:240–248. doi: 10.1016/j.indcrop.2014.06.044. [DOI] [Google Scholar]
- 29.Koba K., Sanda K., Guyon C., Raynaud C., Chaumont J.-P., Nicod L. In vitro cytotoxic activity of Cymbopogon citratus L. and Cymbopogon nardus L. essential oils from Togo. Bangladesh J. Pharmacol. 2008;4:29–34. doi: 10.3329/bjp.v4i1.1040. [DOI] [Google Scholar]
- 30.Kpoviessi S., Bero J., Agbani P., Gbaguidi F., Kpadonou-Kpoviessi B., Sinsin B., Accrombessi G., Frédérich M., Moudachirou M., Quetin-Leclercq J. Chemical composition, cytotoxicity and in vitro antitrypanosomal and antiplasmodial activity of the essential oils of four Cymbopogon species from Benin. J. Ethnopharmacol. 2014;151:652–659. doi: 10.1016/j.jep.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 31.Tofiño-Rivera A., Ortega-Cuadros M., Galvis-Pareja D., Jiménez-Rios H., Merini L.J., Martínez-Pabón M.C. Effect of Lippia alba and Cymbopogon citratus essential oils on biofilms of Streptococcus mutans and cytotoxicity in CHO cells. J. Ethnopharmacol. 2016;194:749–754. doi: 10.1016/j.jep.2016.10.044. [DOI] [PubMed] [Google Scholar]
- 32.Scherer R., Wagner R., Duarte M.C.T., Godoy H.T. Composição e atividades antioxidante e antimicrobiana dos óleos essenciais de cravo-da-índia, citronela e palmarosa. Rev. Bras. Plantas Med. 2009;11:442–449. doi: 10.1590/S1516-05722009000400013. [DOI] [Google Scholar]
- 33.Leite B.L.S., Bonfim R.R., Antoniolli A.R., Thomazzi S.M., Araújo A.A.S., Blank A.F., Estevam C.S., Cambui E.V.F., Bonjardim L.R., Albuquerque Júnior R.L.C., et al. Assessment of antinociceptive, anti-inflammatory and antioxidant properties of Cymbopogon winterianus leaf essential oil. Pharm. Biol. 2010;48:1164–1169. doi: 10.3109/13880200903280000. [DOI] [PubMed] [Google Scholar]
- 34.Silva M.R., Ximenes R.M., da Costa J.G.M., Leal L.K.A.M., de Lopes A.A., de Barros Viana G.S. Comparative anticonvulsant activities of the essential oils (EOs) from Cymbopogon winterianus Jowitt and Cymbopogon citratus (DC) Stapf. in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2010;381:415–426. doi: 10.1007/s00210-010-0494-9. [DOI] [PubMed] [Google Scholar]
- 35.Hasim H., Nasution S.P., Kurniawati S.O., Rachmawati I. Cytotoxic Activity of Citral from Cymbopogon nardus as Anticancer of MCM-B2 Cell. Curr. Biochem. 2020;7:29–36. doi: 10.29244/cb.7.1.4. [DOI] [Google Scholar]
- 36.Sinha S., Jothiramajayam M., Ghosh M., Mukherjee A. Evaluation of toxicity of essential oils palmarosa, citronella, lemongrass and vetiver in human lymphocytes. Food Chem. Toxicol. 2014;68:71–77. doi: 10.1016/j.fct.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 37.Queiroz T.B., Santos G.F., Ventura S.C., Hiruma-Lima C.A., Gaivão I.O.M., Maistro E.L. Cytotoxic and genotoxic potential of geraniol in peripheral blood mononuclear cells and human hepatoma cell line (HepG2) Genet. Mol. Res. 2017;16:1–12. doi: 10.4238/gmr16039777. [DOI] [PubMed] [Google Scholar]
- 38.Gateva S., Jovtchev G., Stankov A., Georgieva A., Dobreva A., Mileva M. The potential of geraniol to reduce cytotoxic and genotoxic effects of MNNG in plant and human lymphocyte test-systems. S. Afr. J. Bot. 2019;123:170–179. doi: 10.1016/j.sajb.2019.03.005. [DOI] [Google Scholar]
- 39.Machado M., Pires P., Dinis A.M., Santos-Rosa M., Alves V., Salgueiro L., Cavaleiro C., Sousa M.C. Monoterpenic aldehydes as potential anti-Leishmania agents: Activity of Cymbopogon citratus and citral on L. infantum, L. tropica and L. major. Exp. Parasitol. 2012;130:223–231. doi: 10.1016/j.exppara.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Santin M.R., dos Santos A.O., Nakamura C.V., Dias Filho B.P., Ferreira I.C.P., Ueda-Nakamura T. In vitro activity of the essential oil of Cymbopogon citratus and its major component (citral) on Leishmania amazonensis. Parasitol. Res. 2009;105:1489–1496. doi: 10.1007/s00436-009-1578-7. [DOI] [PubMed] [Google Scholar]
- 41.Ceole L.F., Cardoso M.D.G., Soares M.J. Nerolidol, the main constituent of Piper aduncum essential oil, has anti- Leishmania braziliensis activity. Parasitology. 2017;144:1179–1190. doi: 10.1017/S0031182017000452. [DOI] [PubMed] [Google Scholar]
- 42.Carneiro J.N.P., Albuquerque R.S., Leite N.F., Machado A.J.T., de Brito D.I.V., Rolón M., Vega C., Coronel C., Coutinho H.D.M., Morais-Braga M.F.B. Avaliação Da Atividade Tripanocida, Leishmanicida e Citotóxica do Geraniol e Citronelal. Cad. Cult. Ciênc. 2015;13:29–36. doi: 10.14295/cad.cult.cienc.v13i2.841. [DOI] [Google Scholar]
- 43.Brito A.M.G. Ph.D. Thesis. Tiradente University; Aracaju, Brazil: 2019. Avaliação da Atividade Antileishmanial dos óleos Essenciais das Plantas Cymbopogon citratus (DC.) Stapf., Eucalyptus citriodora Hook., Mentha arvensis L., e Mentha piperita L. [Google Scholar]
- 44.Essid R., Rahali F.Z., Msaada K., Sghair I., Hammami M., Bouratbine A., Aoun K., Limam F. Antileishmanial and cytotoxic potential of essential oils from medicinal plants in Northern Tunisia. Ind. Crops Prod. 2015;77:795–802. doi: 10.1016/j.indcrop.2015.09.049. [DOI] [Google Scholar]
- 45.Ogungbe I., Setzer W. In-silico Leishmania Target Selectivity of Antiparasitic Terpenoids. Molecules. 2013;18:7761–7847. doi: 10.3390/molecules18077761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vidhya V.M., Dubey V.K., Ponnuraj K. Identification of two natural compound inhibitors of Leishmania donovani Spermidine Synthase (SpdS) through molecular docking and dynamic studies. J. Biomol. Struct. Dyn. 2018;36:2678–2693. doi: 10.1080/07391102.2017.1366947. [DOI] [PubMed] [Google Scholar]
- 47.Youssefi M.R., Moghaddas E., Tabari M.A., Moghadamnia A.A., Hosseini S.M., Farash B.R.H., Ebrahimi M.A., Mousavi N.N., Fata A., Maggi F., et al. In Vitro and In Vivo Effectiveness of Carvacrol, Thymol and Linalool against Leishmania infantum. Molecules. 2019;24:2072. doi: 10.3390/molecules24112072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ueda-Nakamura T., Mendonça-Filho R.R., Morgado-Díaz J.A., Korehisa Maza P., Prado Dias Filho B., Aparício Garcia Cortez D., Alviano D.S., do Rosa M.S.S., Lopes A.H.C.S., Alviano C.S., et al. Antileishmanial activity of Eugenol-rich essential oil from Ocimum gratissimum. Parasitol. Int. 2006;55:99–105. doi: 10.1016/j.parint.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Muhd Haffiz J., Norhayati I., Getha K., Nor Azah M.A., Mohd Ilham A., Lili Sahira H., Roshan Jahn M.S., Muhd Syamil A. Chemical composition and in vitro antitrypanosomal activity of fractions of essential oil from Cymbopogon nardus L. Trop. Biomed. 2013;30:9–14. [PubMed] [Google Scholar]
- 50.Rojas J., Ronceros S., Palacios O., Sevilla C. Efecto anti-Trypanosoma cruzi del aceite esencial de Cymbopogon citratus (DC) Stapf (Hierba luisa) en ratones Balb/c. An. Fac. Med. 2012;73:7. doi: 10.15381/anales.v73i1.803. [DOI] [Google Scholar]
- 51.Costa S., Cavadas C., Cavaleiro C., Salgueiro L., do Céu Sousa M. In vitro susceptibility of Trypanosoma brucei brucei to selected essential oils and their major components. Exp. Parasitol. 2018;190:34–40. doi: 10.1016/j.exppara.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Cardoso J., Soares M.J. In vitro effects of citral on Trypanosoma cruzi metacyclogenesis. Mem. Inst. Oswaldo Cruz. 2010;105:1026–1032. doi: 10.1590/S0074-02762010000800012. [DOI] [PubMed] [Google Scholar]
- 53.Azeredo C.M.O., Soares M.J. Combination of the essential oil constituents citral, eugenol and thymol enhance their inhibitory effect on Crithidia fasciculata and Trypanosoma cruzi growth. Rev. Bras. Farmacogn. 2013;23:762–768. doi: 10.1590/S0102-695X2013000500007. [DOI] [Google Scholar]
- 54.Kouassi E., Coulibaly I., Rodica P., Pintea A., Ouattara S., Odagiu A. HPLC Phenolic Compounds Analysis and Antifungal Activity of extract’s from Cymbopogon citratus (DC) Stapf against Fusarium graminearum and Fusarium oxysporum sp. tulipae. J. Sci. Res. Rep. 2017;14:1–11. doi: 10.9734/JSRR/2017/33810. [DOI] [Google Scholar]
- 55.Molina-Garza Z.J., Bazaldúa-Rodríguez A.F., Quintanilla-Licea R., Galaviz-Silva L. Anti-Trypanosoma cruzi activity of 10 medicinal plants used in northeast Mexico. Acta Trop. 2014;136:14–18. doi: 10.1016/j.actatropica.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 56.Santoro G.F., Cardoso M.G., Guimarães L.G.L., Freire J.M., Soares M.J. Anti-proliferative effect of the essential oil of Cymbopogon citratus (DC) Stapf (lemongrass) on intracellular amastigotes, bloodstream trypomastigotes and culture epimastigotes of Trypanosoma cruzi (Protozoa: Kinetoplastida) Parasitology. 2007;134:1649–1656. doi: 10.1017/S0031182007002958. [DOI] [PubMed] [Google Scholar]
- 57.Moreno É.M., Leal S.M., Stashenko E.E., García L.T. Induction of programmed cell death in Trypanosoma cruzi by Lippia alba essential oils and their major and synergistic terpenes (citral, limonene and caryophyllene oxide) BMC Complement. Altern. Med. 2018;18:225. doi: 10.1186/s12906-018-2293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.dos Anjos D.O., Sobral Alves E.S., Gonçalves V.T., Fontes S.S., Nogueira M.L., Suarez-Fontes A.M., Neves da Costa J.B., Rios-Santos F., Vannier-Santos M.A. Effects of a novel β–lapachone derivative on Trypanosoma cruzi: Parasite death involving apoptosis, autophagy and necrosis. Int. J. Parasitol. Drugs Drug Resist. 2016;6:207–219. doi: 10.1016/j.ijpddr.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimadzu Corporation Gas Chromatography-Mass Spectrometry (GC-MS) [(accessed on 15 April 2022)]. Available online: https://www.shimadzu.eu.com/sites/shimadzu.seg/files/SEG/GCMSBASIC.pdf.
- 60.Shimadzu Corporation Relative Retention. [(accessed on 15 April 2022)]. Available online: https://www.ssi.shimadzu.com/products/gas-chromatography-mass-spectrometry/relative_retention.html.
- 61.Le-Senne A., Muelas-Serrano S., Fernández-Portillo C., Escario J.A., Gómez-Barrio A. Biological characterization of a beta-galactosidase expressing clone of Trypanosoma cruzi CL strain. Mem. Inst. Oswaldo Cruz. 2002;97:1101–1105. doi: 10.1590/S0074-02762002000800006. [DOI] [PubMed] [Google Scholar]
- 62.Roldos V., Nakayama H., Rolón M., Montero-Torres A., Trucco F., Torres S., Vega C., Marrero-Ponce Y., Heguaburu V., Yaluff G. Activity of a hydroxybibenzyl bryophyte constituent against Leishmania spp. and Trypanosoma cruzi: In silico, in vitro and in vivo activity studies. Eur. J. Med. Chem. 2008;43:1797–1807. doi: 10.1016/j.ejmech.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 63.Vega C., Rolón M., Martínez-Fernández A.R., Escario J.A., Gómez-Barrio A. A new pharmacological screening assay with Trypanosoma cruzi epimastigotes expressing beta-galactosidase. Parasitol. Res. 2005;95:296–298. doi: 10.1007/s00436-005-1300-3. [DOI] [PubMed] [Google Scholar]
- 64.Mikus J., Steverding D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue®. Parasitol. Int. 2000;48:265–269. doi: 10.1016/S1383-5769(99)00020-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.