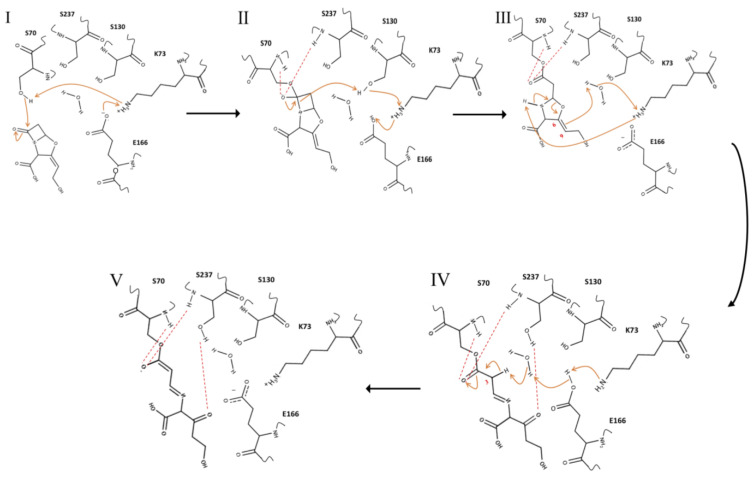
Figure 7.
The proposed mechanism for clavulanic acid inhibition of CTX-M-15. Orange colored lines indicate electron transfer and red dashed lines indicate hydrogen bond interactions. Figures were generated using MarvinSketch v.17.28.0. (Budapest, Hungary). (I) Clavulanic acid binding is displayed as a series proton and electron transfers. Lys73 is deprotonated by Glu166 and then the neutral Lys73 activates Ser70 by abstracting the proton from its sidechain. The nucleophilic Ser70 attacks the b-lactam ring. (II) The tetrahedral transition state is stabilized by an oxyanion hole generated with backbones of Ser70 and Ser237. The bridgehead nitrogen atom abstracts a proton from Ser130, and the shared proton is transferred to Ser130 from Lys73, and then to Lys73 from Glu166. (III) The double bond (C6–C9) in the acyl-enzyme intermediate abstracts a proton from a water molecule. The cleavage of the oxazoline ring is followed by abstracting a proton from a water molecule that is protonated by Lys73, and then neutral Lys73 abstracts a proton from the bridgehead nitrogen atom. (IV) The negatively charged Glu166 abstracts a proton from a water molecule, the activated water molecule removes an acidic hydrogen on the a-carbon (C3). (V) trans-imine-enzyme is stabilized by several hydrogen bond interactions.