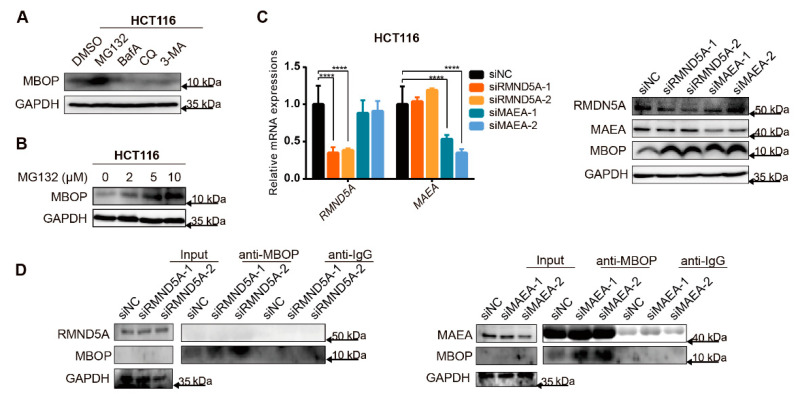
Figure 6.
MBOP is degraded by the ubiquitin–proteasome system. (A) Cells treated with MG132 exhibited higher protein expressions of MBOP compared to other groups, indicating the possibility of involvement of the ubiquitin–protease system in the degradation process of MBOP. (B) The expressions of MBOP and the dose of MG132 showed a positive correlation. (C) Two E3-ligase enzymes RMND5A and MAEA were found in MBOP interacting candidate proteins in Table S4, and the siRNAs targeting RMND5A and MAEA were validated in HCT116. Knockdown of RMND5A and MAEA all decreased the degradation rate of MBOP. Data are presented as mean ± SD, **** p < 0.0001. (D) IP assay showed the direct interaction between MBOP and MAEA, rather than with RMND5A, which indicated that MAEA directly, while RMND5A indirectly, mediated the degradation of MBOP. The expressions of input MBOP in two IP assays were too low to be seen compared to those of the anti-MBOP groups.