Figure 3.
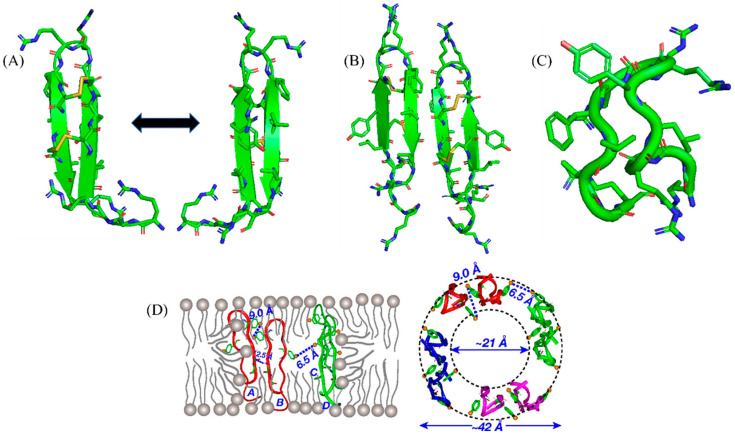
(A) In a solution containing zwitterionic detergent micelle, free PG-1 folds into a typical monomeric beta-hairpin structure [77]. (B) The solid-state NMR analysis demonstrated that PG-1 may create a pore-like shape in a model membrane rich in negatively charged lipids [78]. (C) LPS-bound solution NMR structure of Cys-deleted PG-1 (CDP) [81]. (D) The water-filled pore structure is maintained by the reorganization of oligomeric PG-1 and lipid chains. PG-1-like pore shape was not seen in cholesterol-rich zwitterionic lipid bilayers that mimic eukaryotic membrane compositions [76]. (D) is reproduced from reference 76 Mani, R.; Cady, S.D.; Tang, M.; Waring, A.J.; Lehrer, R.I.; Hong, M. Membrane-dependent oligomeric structure and pore formation of a beta-hairpin antimicrobial peptide in lipid bilayers from solid-state NMR. Proc Natl Acad Sci USA 2006, 103, 16242–16247, doi:10.1073/pnas.0605079103, “Copyright (2006) National Academy of Sciences, USA.”